Susceptibility to experimental infection of the invertebrate locusts (Schistocerca gregaria) with the apicomplexan parasite Neospora caninum
- PMID: 25493211
- PMCID: PMC4260130
- DOI: 10.7717/peerj.674
Susceptibility to experimental infection of the invertebrate locusts (Schistocerca gregaria) with the apicomplexan parasite Neospora caninum
Abstract
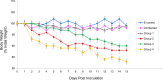
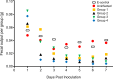
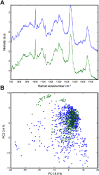
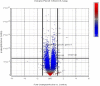
Neuropathogenesis is a feature of Neospora caninum infection. In order to explore this in the absence of acquired host immunity to the parasite, we have tested infection in locusts (Schistocerca gregaria). We show for the first time that locusts are permissive to intra-hemocoel infection with N. caninum tachyzoites. This was characterized by alteration in body weight, fecal output, hemoparasitemia, and sickness-related behavior. Infected locusts exhibited progressive signs of sickness leading to mortality. Also, N. caninum showed neuropathogenic affinity, induced histological changes in the brain and was able to replicate in the brain of infected locusts. Fatty acid (FA) profiling analysis of the brains by gas chromatography and multi-variate prediction models discriminated with high accuracy (98%) between the FA profiles of the infected and control locusts. DNA microarray gene expression profiling distinguished infected from control S. gregaria brain tissues on the basis of distinct differentially-expressed genes. These data indicate that locusts are permissible to infection with N. caninum and that the parasite retains its tropism for neural tissues in the invertebrate host. Locusts may facilitate preclinical testing of interventional strategies to inhibit the growth of N. caninum tachyzoites. Further studies on how N. caninum brings about changes in locust brain tissue are now warranted.
Keywords: Behaviour; Host-pathogen interaction; Infection; Invertebrate model; Locusts.
Figures





Similar articles
-
Neospora caninum infection induces an isolate virulence-dependent pro-inflammatory gene expression profile in bovine monocyte-derived macrophages.Parasit Vectors. 2020 Jul 25;13(1):374. doi: 10.1186/s13071-020-04239-3. Parasit Vectors. 2020. PMID: 32711550 Free PMC article.
-
Differential susceptibility of human trophoblastic (BeWo) and uterine cervical (HeLa) cells to Neospora caninum infection.Int J Parasitol. 2010 Dec;40(14):1629-37. doi: 10.1016/j.ijpara.2010.06.010. Epub 2010 Aug 12. Int J Parasitol. 2010. PMID: 20708622
-
RNA sequencing reveals dynamic expression of lncRNAs and mRNAs in caprine endometrial epithelial cells induced by Neospora caninum infection.Parasit Vectors. 2022 Aug 24;15(1):297. doi: 10.1186/s13071-022-05405-5. Parasit Vectors. 2022. PMID: 35999576 Free PMC article.
-
Tissue culture and explant approaches to studying and visualizing Neospora caninum and its interactions with the host cell.Microsc Microanal. 2004 Oct;10(5):602-20. doi: 10.1017/S1431927604040930. Microsc Microanal. 2004. PMID: 15525434 Review.
-
The antigenic composition of Neospora caninum.Int J Parasitol. 1999 Aug;29(8):1175-88. doi: 10.1016/s0020-7519(99)00085-5. Int J Parasitol. 1999. PMID: 10576569 Review.
References
-
- Bacardit J, Burke EK, Krasnogor N. Improving the scalability of rule-based evolutionary learning. Memetic Computing. 2009;1(1):55–67. doi: 10.1007/s12293-008-0005-4. - DOI
-
- Badisco L, Huybrechts J, Simonet G, Verlinden H, Marchal E, Huybrechts R, Schoofs L, De Loof A, Vanden Broeck J. Transcriptome analysis of the desert locust central nervous system: production and annotation of a Schistocerca gregaria EST Database. PLoS ONE. 2011;6(3):e674. doi: 10.1371/journal.pone.0017274. - DOI - PMC - PubMed
-
- Bartley PM, Katzer F, Rocchi MS, Maley SW, Benavides J, Nath M, Pang Y, Cantón G, Thomson J, Chianini F, Innes EA. Development of maternal and foetal immune responses in cattle following experimental challenge with Neospora caninum at day 210 of gestation. Veterinary Research. 2013;44:91. doi: 10.1186/1297-9716-44-91. - DOI - PMC - PubMed
Grants and funding
- BB/E01772X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/B/1356X/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I001271/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E022758/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G17764/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

