Increased CXCL10 expression in MS MSCs and monocytes is unaffected by AHSCT
- PMID: 25493279
- PMCID: PMC4241792
- DOI: 10.1002/acn3.92
Increased CXCL10 expression in MS MSCs and monocytes is unaffected by AHSCT
Abstract
Objective: To confirm CXCL10 over production in bone marrow mesenchymal stem cells (MSCs) and circulating monocytes isolated from multiple sclerosis patients (MS) and identify predate cell molecular signature; to extend this analysis after autologous hematopoietic stem cell transplantation (AHSCT) to test if therapy has modifying effects on MSCs and circulating monocytes.
Methods: MSCs and monocytes were isolated from 19 MS patients who undergone AHSCT before and seven of them at least 3 years after transplant. CXCL10 production was detected after LPS/IFN-γ stimulation. TLR4 signaling pathways were investigated by means of transcription factors phosphorylation/activation level. RT-PCR of activated transcription factors was performed to quantify their expression. All experiments were conducted in parallel with 24 matched healthy donors (HD).
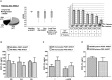
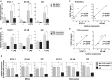
Results: CXCL10 expression was significantly increased in both peripheral circulating monocytes and BM MSCs compared to HD. We showed that CXCL10 production is determined by an altered signaling pathway downstream TLR4, with the involvement of STAT-1, NF-κB, p38, JNK, and CREB. All upregulated transcription factors are more phosphorylated in MS patient sample. These features are not modified after AHSCT.
Interpretation: We demonstrated that in MS two different cell lineages are characterized by significantly increased production of CXCL10, due to altered signaling pathways of innate immune reaction mediated by TLR4, probably associated with disease phenotype. This characteristic is not modified by AHSCT, suggesting that when T and B lymphocytes are reset, other possible components of MS pathology, such as CXCL10 over production, do not determine therapy outcome.
Figures


References
-
- Casatella MA, Gasperini S, Calzetti F, et al. Regulated production of the interferon-γ-inducible protein−10 (IP-10) chemokine by human neutrophils. Eur J Immunol. 1997;27:111–115. - PubMed
-
- Mazzanti B, Aldinucci A, Biagioli T, et al. Differences in mesenchymal stem cells cytokine profiles between MS patients and healthy donors: implication for assessment of disease activity and treatment. J Neuroimmunol. 2008;199:142–150. - PubMed
-
- Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7:626–636. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

