Subplate in a rat model of preterm hypoxia-ischemia
- PMID: 25493282
- PMCID: PMC4241795
- DOI: 10.1002/acn3.97
Subplate in a rat model of preterm hypoxia-ischemia
Abstract
Objective: Hypoxia-ischemia (HI) in preterm infants primarily leads to injuries in the cerebral white matter. However, there is growing evidence that perinatal injury in preterms can also involve other zones including the cortical gray matter. In a neonatal rat model of HI, selective vulnerability of subplate has been suggested using BrdU birth-dating methods. In this study, we aimed to investigate the neuropathological changes of the subplate and deep layers of the cortex following cerebral HI in neonatal rats with specific cell markers.
Methods: P2 rats underwent permanent occlusion of the right common carotid artery followed by a period of hypoxia. P8 rats were analyzed using immunohistochemistry; subplate and deep layers cells were quantified and compared with sham-operated case.
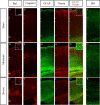
Results: A large variability in the extent of the cerebral injury was apparent. For the three analyzed subplate populations (Nurr1+, Cplx3+, and Ctgf+ cells), no significant cell reduction was observed in mild and moderate cases. Only in severe cases, subplate cells were strongly affected, but these injuries were always accompanied by the cell reductions in layers VI and V.
Interpretation: We could therefore not confirm a specific vulnerability of subplate cells compared to other deep layers or the white matter in our model.
Figures




References
-
- Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Semin Neonatal. 2000;5:3–16. - PubMed
-
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79:181–188. - PubMed
-
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. - PubMed
-
- Kostović I, Judaš M, Radoš M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

