Programmed necrosis in the cross talk of cell death and inflammation
- PMID: 25493335
- PMCID: PMC4394030
- DOI: 10.1146/annurev-immunol-032414-112248
Programmed necrosis in the cross talk of cell death and inflammation
Abstract
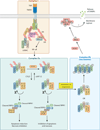
Cell proliferation and cell death are integral elements in maintaining homeostatic balance in metazoans. Disease pathologies ensue when these processes are disturbed. A plethora of evidence indicates that malfunction of cell death can lead to inflammation, autoimmunity, or immunodeficiency. Programmed necrosis or necroptosis is a form of nonapoptotic cell death driven by the receptor interacting protein kinase 3 (RIPK3) and its substrate, mixed lineage kinase domain-like (MLKL). RIPK3 partners with its upstream adaptors RIPK1, TRIF, or DAI to signal for necroptosis in response to death receptor or Toll-like receptor stimulation, pathogen infection, or sterile cell injury. Necroptosis promotes inflammation through leakage of cellular contents from damaged plasma membranes. Intriguingly, many of the signal adaptors of necroptosis have dual functions in innate immune signaling. This unique signature illustrates the cooperative nature of necroptosis and innate inflammatory signaling pathways in managing cell and organismal stresses from pathogen infection and sterile tissue injury.
Keywords: DAI; MCMV; MLKL; RIPK1; RIPK3; TNF; TRIF; inflammation; necroptosis; vaccinia virus.
Figures




References
-
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. - PubMed
-
- Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011;411:569–573. - PubMed
-
- Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

