First steps toward harmonized human biomonitoring in Europe: demonstration project to perform human biomonitoring on a European scale
- PMID: 25493439
- PMCID: PMC4348748
- DOI: 10.1289/ehp.1408616
First steps toward harmonized human biomonitoring in Europe: demonstration project to perform human biomonitoring on a European scale
Abstract
Background: For Europe as a whole, data on internal exposure to environmental chemicals do not yet exist. Characterization of the internal individual chemical environment is expected to enhance understanding of the environmental threats to health.
Objectives: We developed and applied a harmonized protocol to collect comparable human biomonitoring data all over Europe.
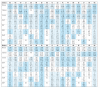
Methods: In 17 European countries, we measured mercury in hair and cotinine, phthalate metabolites, and cadmium in urine of 1,844 children (5-11 years of age) and their mothers. Specimens were collected over a 5-month period in 2011-2012. We obtained information on personal characteristics, environment, and lifestyle. We used the resulting database to compare concentrations of exposure biomarkers within Europe, to identify determinants of exposure, and to compare exposure biomarkers with health-based guidelines.
Results: Biomarker concentrations showed a wide variability in the European population. However, levels in children and mothers were highly correlated. Most biomarker concentrations were below the health-based guidance values.
Conclusions: We have taken the first steps to assess personal chemical exposures in Europe as a whole. Key success factors were the harmonized protocol development, intensive training and capacity building for field work, chemical analysis and communication, as well as stringent quality control programs for chemical and data analysis. Our project demonstrates the feasibility of a Europe-wide human biomonitoring framework to support the decision-making process of environmental measures to protect public health.
Conflict of interest statement
E.D.H., E.G., H.W., R.S., G.K., and G.S. are employed by the Unit Environmental Risk and Health, VITO, Mol, Belgium, a public nonprofit research institute that receives core funding from the Flemish authorities and in-part funding through contracts from the public and private sector. L.B. is employed by Environmental Health Sciences International, Hulst, the Netherlands, a consulting company that has not consulted for industry during the last 3 years. A.J. and R.J. are employed by BiPRO GmbH, Munich, Germany, a consulting company for the public and private sectors. A.C.G. is employed by the Luxembourg Institute of Science and Technology (LIST), Belvaux, Luxembourg, a public nonprofit research institute that receives core funding from the Luxembourg authorities and partial funding through contracts from the public and private sectors. D.L. and M.J. are employed by the Nofer Institute of Occupational Medicine, Lodz, Poland, a scientific research center that deals with issues related to public health, environmental health, and occupational medicine and receives core funding from the Polish Ministry of Health and Ministry of Science and Higher Education and in-part funding from the public and private sectors for analyses made by ISO 17025–accredited laboratories or expertise-specific GLP laboratories. M.F.R. and S.N. are employed by the Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal, a public academic and research institute that receives partial funding from industrial contracts. A.E.G. and I.-R.L. are employed by the Environmental Health Center, Cluj-Napoca, Romania, which offers consulting services to industry but does not receive funding from industry. These authors declare that no private funding was used to perform work related to this manuscript. The other authors declare they have no actual or potential competing financial interests.
Figures

Comment in
-
Continental reference point: harmonized human biomonitoring across Europe.Environ Health Perspect. 2015 Mar;123(3):A71. doi: 10.1289/ehp.123-A71. Environ Health Perspect. 2015. PMID: 25730104 Free PMC article. No abstract available.
References
-
- Akerstrom M, Barregard L, Lundh T, Sallsten G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol. 2013;268:286–293. - PubMed
-
- Angerer J, Bird MG, Burke TA, Doerrer NG, Needham L, Robison SH, et al. Strategic biomonitoring initiatives: moving the science forward. Toxicol Sci. 2006;93:3–10. - PubMed
-
- Aylward LL, Hays SM, Gagné M, Krishnan K. Derivation of Biomonitoring Equivalents for di(2-ethylhexyl)phthalate (CAS No. 117-81-7). Regul Toxicol Pharmacol. 2009a;55:249–258. - PubMed
-
- Aylward LL, Hays SM, Gagné M, Krishnan K. Derivation of Biomonitoring Equivalents for di-n-butyl phthalate (DBP), benzylbutyl phthalate (BzBP), and diethyl phthalate (DEP). Regul Toxicol Pharmacol. 2009b;55:259–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

