Dose-Dependent AMPK-Dependent and Independent Mechanisms of Berberine and Metformin Inhibition of mTORC1, ERK, DNA Synthesis and Proliferation in Pancreatic Cancer Cells
- PMID: 25493642
- PMCID: PMC4262417
- DOI: 10.1371/journal.pone.0114573
Dose-Dependent AMPK-Dependent and Independent Mechanisms of Berberine and Metformin Inhibition of mTORC1, ERK, DNA Synthesis and Proliferation in Pancreatic Cancer Cells
Abstract
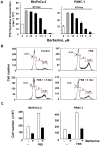
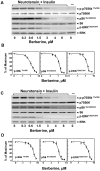
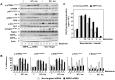
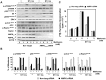
Natural products represent a rich reservoir of potential small chemical molecules exhibiting anti-proliferative and chemopreventive properties. Here, we show that treatment of pancreatic ductal adenocarcinoma (PDAC) cells (PANC-1, MiaPaCa-2) with the isoquinoline alkaloid berberine (0.3-6 µM) inhibited DNA synthesis and proliferation of these cells and delay the progression of their cell cycle in G1. Berberine treatment also reduced (by 70%) the growth of MiaPaCa-2 cell growth when implanted into the flanks of nu/nu mice. Mechanistic studies revealed that berberine decreased mitochondrial membrane potential and intracellular ATP levels and induced potent AMPK activation, as shown by phosphorylation of AMPK α subunit at Thr-172 and acetyl-CoA carboxylase (ACC) at Ser79. Furthermore, berberine dose-dependently inhibited mTORC1 (phosphorylation of S6K at Thr389 and S6 at Ser240/244) and ERK activation in PDAC cells stimulated by insulin and neurotensin or fetal bovine serum. Knockdown of α1 and α2 catalytic subunit expression of AMPK reversed the inhibitory effect produced by treatment with low concentrations of berberine on mTORC1, ERK and DNA synthesis in PDAC cells. However, at higher concentrations, berberine inhibited mitogenic signaling (mTORC1 and ERK) and DNA synthesis through an AMPK-independent mechanism. Similar results were obtained with metformin used at doses that induced either modest or pronounced reductions in intracellular ATP levels, which were virtually identical to the decreases in ATP levels obtained in response to berberine. We propose that berberine and metformin inhibit mitogenic signaling in PDAC cells through dose-dependent AMPK-dependent and independent pathways.
Conflict of interest statement
Figures


Similar articles
-
Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells.PLoS One. 2013;8(2):e57289. doi: 10.1371/journal.pone.0057289. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437362 Free PMC article.
-
Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: dependence on glucose concentration and role of AMPK.Biochem Biophys Res Commun. 2013 Jan 4;430(1):352-7. doi: 10.1016/j.bbrc.2012.11.010. Epub 2012 Nov 15. Biochem Biophys Res Commun. 2013. PMID: 23159620 Free PMC article.
-
Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth.Cancer Res. 2009 Aug 15;69(16):6539-45. doi: 10.1158/0008-5472.CAN-09-0418. Cancer Res. 2009. PMID: 19679549 Free PMC article.
-
Abilities of berberine and chemically modified berberines to interact with metformin and inhibit proliferation of pancreatic cancer cells.Adv Biol Regul. 2019 Aug;73:100633. doi: 10.1016/j.jbior.2019.04.003. Epub 2019 Apr 21. Adv Biol Regul. 2019. PMID: 31047842 Review.
-
AMPK--sensing energy while talking to other signaling pathways.Cell Metab. 2014 Dec 2;20(6):939-52. doi: 10.1016/j.cmet.2014.09.013. Epub 2014 Oct 30. Cell Metab. 2014. PMID: 25448702 Free PMC article. Review.
Cited by
-
Triciribine increases LDLR expression and LDL uptake through stabilization of LDLR mRNA.Sci Rep. 2018 Nov 1;8(1):16174. doi: 10.1038/s41598-018-34237-6. Sci Rep. 2018. PMID: 30385871 Free PMC article.
-
Obesity and Pancreatic Cancer: Insight into Mechanisms.Cancers (Basel). 2021 Oct 10;13(20):5067. doi: 10.3390/cancers13205067. Cancers (Basel). 2021. PMID: 34680216 Free PMC article. Review.
-
Low dosage combination treatment with metformin and simvastatin inhibits obesity-promoted pancreatic cancer development in male KrasG12D mice.Sci Rep. 2023 Sep 26;13(1):16144. doi: 10.1038/s41598-023-43498-9. Sci Rep. 2023. PMID: 37752238 Free PMC article.
-
Antiviral activity of berberine.Arch Virol. 2020 Sep;165(9):1935-1945. doi: 10.1007/s00705-020-04706-3. Epub 2020 Jun 28. Arch Virol. 2020. PMID: 32594322 Free PMC article. Review.
-
Effects of Berberine against Pancreatitis and Pancreatic Cancer.Molecules. 2022 Dec 6;27(23):8630. doi: 10.3390/molecules27238630. Molecules. 2022. PMID: 36500723 Free PMC article. Review.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, et al. (2014) Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res 74:2913–2921. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA: A Cancer Journal for Clinicians 64:9–29. - PubMed
-
- Brand RE, Tempero MA (1998) Pancreatic cancer. Curr Opin Oncol 10:362–366. - PubMed
-
- Rozengurt E, Walsh JH (2001) Gastrin, CCK, signaling, and cancer. Annu Rev Physiol 63:49–76. - PubMed
-
- Rozengurt E (2002) Neuropeptides as growth factors for normal and cancer cells. Trends Endocrinol Metabol 13:128–134. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

