Cobalt(III)-catalyzed synthesis of indazoles and furans by C-H bond functionalization/addition/cyclization cascades
- PMID: 25494296
- PMCID: PMC4304451
- DOI: 10.1021/ja5116452
Cobalt(III)-catalyzed synthesis of indazoles and furans by C-H bond functionalization/addition/cyclization cascades
Abstract
The development of operationally straightforward and cost-effective routes for the assembly of heterocycles from simple inputs is important for many scientific endeavors, including pharmaceutical, agrochemical, and materials research. In this article we describe the development of a new air-stable cationic Co(III) catalyst for convergent, one-step benchtop syntheses of N-aryl-2H-indazoles and furans by C-H bond additions to aldehydes followed by in situ cyclization and aromatization. Only a substoichiometric amount of AcOH is required as an additive that is both low-cost and convenient to handle. The syntheses of these heterocycles are the first examples of Co(III)-catalyzed additions to aldehydes, and reactions are demonstrated for a variety of aromatic, heteroaromatic, and aliphatic derivatives. The syntheses of both N-aryl-2H-indazoles and furans have been performed on 20 mmol scales and should be readily applicable to larger scales. The reported heterocycle syntheses also demonstrate the use of directing groups that have not previously been applied to Co(III)-catalyzed C-H bond functionalizations. Additionally, the synthesis of furans demonstrates the first example of Co(III)-catalyzed functionalization of alkenyl C-H bonds.
Figures





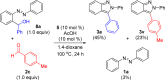

References
-
-
For select recent reviews on C–H functionalization, see:
- Wencel-Delord J.; Glorius F. Nat. Chem. 2013, 5, 369. - PubMed
- Arockiam P. B.; Bruneau C.; Dixneuf P. H. Chem. Rev. 2012, 112, 5879. - PubMed
- Li B.-J.; Shi Z.-J. Chem. Soc. Rev. 2012, 41, 5588. - PubMed
- Yamaguchi J.; Yamaguchi A. D.; Itami K. Angew. Chem., Int. Ed. 2012, 51, 8960. - PubMed
- Engle K. M.; Mei T.-S.; Wasa M.; Yu J.-Q. Acc. Chem. Res. 2012, 45, 788. - PMC - PubMed
- Ackermann L. Chem. Rev. 2011, 111, 1315. - PubMed
- Cho S. H.; Kim J. Y.; Kwak J.; Chang S. Chem. Soc. Rev. 2011, 40, 5068. - PubMed
- Lyons T. W.; Sanford M. S. Chem. Rev. 2010, 110, 1147. - PMC - PubMed
- Mkhalid I. A. I.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. Chem. Rev. 2010, 110, 890. - PubMed
- Colby D. A.; Bergman R. G.; Ellman J. A. Chem. Rev. 2010, 110, 624. - PMC - PubMed
- Seregin I. V.; Gevorgyan V. Chem. Soc. Rev. 2007, 36, 1173. - PMC - PubMed
-
-
-
For reviews on C–H bond additions to polarized π bonds, see:
- Zhang X.-S.; Chen K.; Shi Z.-J. Chem. Sci. 2014, 5, 2146.
- Yan G.; Wu X.; Yang M. Org. Biomol. Chem. 2013, 11, 5558. - PubMed
-
-
-
For examples of Rh(III)-catalyzed C–H bond additions to destabilized, highly electron-deficient aldehydes to provide alcohols, see:
- Li Y.; Zhang X.-S.; Chen K.; He K.-H.; Pan F.; Li B.-J.; Shi Z.-J. Org. Lett. 2012, 14, 636. - PubMed
- Li Y.; Zhang X.-S.; Zhu Q.-L.; Shi Z.-J. Org. Lett. 2012, 14, 4498. - PubMed
- Yang L.; Correia C. A.; Li C.-J. Adv. Synth. Catal. 2011, 353, 1269.
-
-
-
For examples of Ir-, Re-, and Mn-catalyzed C–H bond addition to aldehydes with capture of alcohol products as silyl ethers, see:
- Li B.-J.; Shi Z.-J. Chem. Sci. 2011, 2, 488.
- Kuninobu Y.; Asanoma D.; Takai K. Synlett 2010, 2883.
- Kuninobu Y.; Fujii Y.; Matsuki T.; Nishina Y.; Takai K. Org. Lett. 2009, 11, 2711. - PubMed
- Kuninobu Y.; Nishina Y.; Takeuchi T.; Takai K. Angew. Chem., Int. Ed. 2007, 46, 6518. - PubMed
- Fukumoto Y.; Sawada K.; Hagihara M.; Chatani N.; Murai S. Angew. Chem., Int. Ed. 2002, 41, 2779. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

