Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes
- PMID: 25494452
- PMCID: PMC4351578
- DOI: 10.1164/rccm.201410-1771OC
Plasma sphingolipids associated with chronic obstructive pulmonary disease phenotypes
Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) occurs in a minority of smokers and is characterized by intermittent exacerbations and clinical subphenotypes such as emphysema and chronic bronchitis. Although sphingolipids as a class are implicated in the pathogenesis of COPD, the particular sphingolipid species associated with COPD subphenotypes remain unknown.
Objectives: To use mass spectrometry to determine which plasma sphingolipids are associated with subphenotypes of COPD.
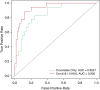
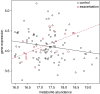
Methods: One hundred twenty-nine current and former smokers from the COPDGene cohort had 69 distinct sphingolipid species detected in plasma by targeted mass spectrometry. Of these, 23 were also measured in 131 plasma samples (117 independent subjects) using an untargeted platform in an independent laboratory. Regression analysis with adjustment for clinical covariates, correction for false discovery rate, and metaanalysis were used to test associations between COPD subphenotypes and sphingolipids. Peripheral blood mononuclear cells were used to test associations between sphingolipid gene expression and plasma sphingolipids.
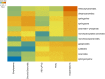
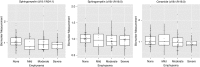
Measurements and main results: Of the measured plasma sphingolipids, five sphingomyelins were associated with emphysema; four trihexosylceramides and three dihexosylceramides were associated with COPD exacerbations. Three sphingolipids were strongly associated with sphingolipid gene expression, and 15 sphingolipid gene/metabolite pairs were differentially regulated between COPD cases and control subjects.
Conclusions: There is evidence of systemic dysregulation of sphingolipid metabolism in patients with COPD. Subphenotyping suggests that sphingomyelins are strongly associated with emphysema and glycosphingolipids are associated with COPD exacerbations.
Keywords: ceramides; emphysema; genomics; metabolomics; sphingomyelins.
Figures

Comment in
-
Bioactive lipids in emphysema. Decoding fat to reveal COPD phenotypes.Am J Respir Crit Care Med. 2015 Feb 1;191(3):241-3. doi: 10.1164/rccm.201412-2264ED. Am J Respir Crit Care Med. 2015. PMID: 25635483 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL089897/HL/NHLBI NIH HHS/United States
- T15 LM009451/LM/NLM NIH HHS/United States
- P20 HL-113444/HL/NHLBI NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- P20 HL113444/HL/NHLBI NIH HHS/United States
- R01 HL077328/HL/NHLBI NIH HHS/United States
- R01 HL089856/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- R01 HL129937/HL/NHLBI NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- P20 HL-11344/HL/NHLBI NIH HHS/United States
- P20 HL113445/HL/NHLBI NIH HHS/United States
- S10 RR023703/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

