Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS): study protocol for a randomized controlled trial
- PMID: 25494702
- PMCID: PMC4301403
- DOI: 10.1186/1745-6215-15-488
Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS): study protocol for a randomized controlled trial
Abstract
Background: Medication regimens for asthma are particularly vulnerable to adherence problems because of the requirement for long-term use and periods of symptom remission experienced by patients. Pharmacists are suited to impact medication adherence given their training, skills, and frequent contact with patients. The Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS) trial involves an intervention leveraging mobile health (mHealth) technology to support community pharmacy practice with the hypothesis of improved medication adherence in asthma.
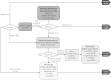
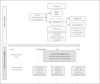
Methods/design: This study is a pragmatic pharmacy-based, cluster, randomized controlled trial with 12 months of intervention delivery and follow-up. Pharmacies (the clusters) will be randomized at a 1:1 ratio to provide intervention or usual care. The EmPhAsIS intervention consists of patient asthma education, short message service (SMS)-based monthly assessment of adherence, and follow-up of non-adherent individuals by community pharmacists. There are no inclusion or exclusion criteria for pharmacies. Patients are eligible if they: are 14 years of age or older, fill a prescription for inhaled corticosteroid (either monotherapy or in a combination inhaler with long-acting beta-agonists), have been diagnosed with asthma, possess a mobile phone with SMS capabilities, and have no communication difficulties such as inability to communicate in English, or significant impairment in vision, hearing, or speech. The primary outcome is adherence to inhaled corticosteroids ascertained by the medication possession ratio, the ratio of the days of medication supplied to days in a given time interval. This study will also evaluate secondary outcomes including: asthma control, asthma-related quality of life, asthma-related hospital admissions, and use of reliever medications during the follow-up period. A nested economic evaluation using a probabilistic decision-analytic model will be used to perform a cost-effectiveness analysis from the societal perspective of the intervention compared with usual care over a 10-year time horizon.
Discussion: Considering the prevalence of asthma, the extent of the non-adherence problem in this disease, and the availability of effective treatments, there is a tremendous potential to reduce the burden of asthma through improving adherence. This is the first study of an intervention based on mobile communication technology involving community pharmacists in asthma management.
Trial registration: ClinicalTrials.gov identifier: NCT02170883; date of registration: 19 June 2014.
Figures
References
-
- Statistics Canada: Asthma, by Sex, Provinces and Territories. Available from: http://www.statcan.gc.ca/pub/82-625-x/2011001/article/11458-eng.htm
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

