Endo-selective Pd-catalyzed silyl methyl Heck reaction
- PMID: 25494921
- PMCID: PMC4291758
- DOI: 10.1021/ja5104525
Endo-selective Pd-catalyzed silyl methyl Heck reaction
Abstract
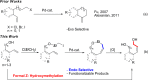
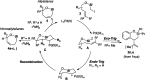
A palladium (Pd)-catalyzed endo-selective Heck reaction of iodomethylsilyl ethers of phenols and aliphatic alkenols has been developed. Mechanistic studies reveal that this silyl methyl Heck reaction operates via a hybrid Pd-radical process and that the silicon atom is crucial for the observed endo selectivity. The obtained allylic silyloxycycles were further oxidized into (Z)-alkenyldiols.
Figures

References
-
-
For review of the Heck reaction, see:
- The Mizoroki-Heck Reaction; Oestreich M., Ed.; John Wiley & Sons: West Sussex, U.K., 2009.
-
-
- Firmansjah L.; Fu G. C. J. Am. Chem. Soc. 2007, 129, 11340. - PubMed
-
- Bloome K. S.; McMahen R. L.; Alexanian E. J. J. Am. Chem. Soc. 2011, 133, 20146. - PubMed
-
-
For carbonylative radical Heck-type reactions of alkyl iodides, see:
- Bloome K. S.; Alexanian E. J. J. Am. Chem. Soc. 2010, 132, 12823. - PubMed
-
For radical cobalt-catalyzed coupling of alkyl iodides with alkenes, see:
- Weiss M. E.; Kreis L. M.; Lauber A.; Carreira E. M. Angew. Chem., Int. Ed. 2011, 50, 11125. - PubMed
-
For intermolecular radical Heck reactions of alkyl halides, see:
- Zou Y.; Zhou J. Chem. Commun. 2014, 50, 3725. - PubMed
- McMahon C. M.; Alexanian E. J. Angew. Chem., Int. Ed. 2014, 53, 5974. - PubMed
-
-
- Hegedus L. S.; Sestrick M. R.; Michaelson E. T.; Harrington P. J. J. Org. Chem. 1989, 54, 4141.
- Owczarczyk Z.; Lamaty F.; Vawter E. J.; Negishi E. J. Am. Chem. Soc. 1992, 114, 10091.
- Rigby J. H.; Hughes R. C.; Heeg M. J. J. Am. Chem. Soc. 1995, 117, 7834.
- Iimura S.; Overman L. E.; Paulini R.; Zakarian A. J. Am. Chem. Soc. 2006, 128, 13095. - PMC - PubMed
- Klein J. E. M. N.; Muller-Bunz H.; Ortin Y.; Evans P. Tetrahedron Lett. 2008, 49, 7187.
- Kim S. H.; Lee S.; Lee H. S.; Kim J. N. Tetrahedron Lett. 2010, 51, 6305.
- Kotoku N.; Sumii Y.; Kobayashi M. Org. Lett. 2011, 13, 3514. - PubMed
- Gao P.; Cook S. P. Org. Lett. 2012, 14, 3340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

