Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study
- PMID: 25494967
- PMCID: PMC4318899
- DOI: 10.1210/jc.2014-3014
Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves' orbitopathy: a randomized controlled study
Abstract
Background: Preliminary studies have shown that rituximab (RTX) is effective in the treatment of active Graves' orbitopathy (GO).
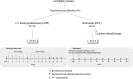
Methods: We conducted a double-blind, randomized trial (European Clinical Trials Database [EudraCT] 2007-003910-33) to compare RTX with iv methylprednisolone (ivMP) in patients with active moderate to severe GO. Thirty-two patients were randomized to receive either ivMP (7.5 g) or RTX (2000 or 500 mg). The primary end point was the decrease of the clinical activity score of 2 points or to less than 3 at week 24. Changes of proptosis, lid fissure, diplopia and eye muscle motility, and quality of life score were secondary end points. The number of therapeutic responses, disease reactivation, and surgical procedures required during follow-up and the patients' quality of life were also assessed.
Results: The clinical activity score decreased with both treatments but more after RTX at 16, 20, and 24 weeks (P < .04, P < .02, P < .006, respectively), whether 1000 mg RTX twice or 500 mg RTX once was used (P = NS). At 24 weeks 100% of RTX patients improved compared with 69% after ivMP (P < .001). Disease reactivation was never observed in RTX patients but was observed in five after ivMP. Patients treated with RTX scored better motility at 52 weeks in both the right (P = .014) and the left eye (P = .026). Overall rehabilitative surgical procedures carried out during follow-up (at 76 wk) were 12 in 16 ivMP patients and 5 in 15 RTX patients (P = .049).
Conclusions: The results of this trial confirm preliminary reports on a better therapeutic outcome of RTX in active moderate to severe GO, when compared with ivMP, even after a lower RTX dose. The better eye motility outcome, visual functioning of the quality of life assessment, and the reduced number of surgical procedures in patients after RTX seem to suggest a disease-modifying effect of the drug.
Figures



References
-
- Daumerie C. Epidemiology. In: Wiersinga WM, Kahaly G, eds. Graves' Orbitopathy: A Multidisciplinary Approach. 2nd ed Basel, Switzerland: Karger; 2010:33–38.
-
- Bartalena L, Krassas GE, Wiersinga W, et al. European Group on Graves' Orbitopathy. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–4463. - PubMed
-
- Vannucchi G, Covelli D, Campi I, et al. The therapeutic outcome to intravenous steroid therapy for active Graves' orbitopathy is influenced by the time of response but not polymorphisms of the glucocorticoid receptor. Eur J Endocrinol. 2013;170:55–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

