Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres
- PMID: 25495212
- PMCID: PMC6496866
- DOI: 10.1111/cpr.12156
Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres
Abstract
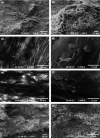
Objectives: To compare potential of four types of stem cell in tissue engineering and regenerative medicine applications, osteogenic capacity of newly introduced mesenchymal stem cells (MSCs) derived from buccal fat pads (BFP) (an adipose-encapsulated mass of the oral cavity), was compared to those isolated from bone marrow (BM-MSCs), adipose tissue (AT-MSCs) and unrestricted somatic stem cells (USSCs). Cells were cultured on poly (L-lactide) (PLLA) nanofibres, Bio-Oss(®)-coated PLLA (PLLA-Bio), and culture plates (TCPS) as control.
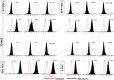
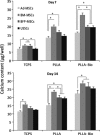
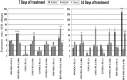
Materials and methods: Capacity of proliferation and osteogenic differentiation of the stem cells was investigated by MTT assay and common osteogenic markers, alkaline phosphatase activity, calcium mineral deposition and bone-related genes.
Results: Highest proliferation level was observed in cells cultured on PLLA-Bio, but with no significant difference between proliferation levels of the four types of stem cell. Over the period of study, BM-MSCs cultured on PLLA-Bio scaffolds exhibited greatest alkaline phosphatase (ALP) activity and mineralization with BFP-MSCs having the next closest results. However, AT-MSC had the lowest capacity for ALP activity and mineralization during osteogenic differentiation. Gene expression evaluation revealed that highest expression of three important bone-related genes was observed in stem cells cultured on bioceramic-coated nanofibrous scaffolds.
Conclusions: Results indicated Bio-Oss-coated PLLA to compose most appropriate substrates to support proliferation and osteogenic differentiation of stem cells in vitro. BFP-MSCs demonstrated the same osteogenic differentiation capacity as other stem cells tested and thus hold very promising potential for applications in bone tissue engineering and regenerative medicine.
© 2014 John Wiley & Sons Ltd.
Figures





References
-
- Caplan AI (2005) Review: mesenchymal stem cells: cell‐based reconstructive therapy in orthopedics. Tissue Eng. 11, 1198–1211. - PubMed
-
- Koç ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R et al (1999) Bone marrow–derived mesenchymal stem cells remain host‐derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp. Hematol. 27, 1675–1681. - PubMed
-
- DiGirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ (1999) Propagation and senescence of human marrow stromal cells in culture: a simple colony‐forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 107, 275–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

