Huntington's Disease (HD): Neurodegeneration of Brodmann's Primary Visual Area 17 (BA17)
- PMID: 25495445
- PMCID: PMC8029049
- DOI: 10.1111/bpa.12237
Huntington's Disease (HD): Neurodegeneration of Brodmann's Primary Visual Area 17 (BA17)
Abstract
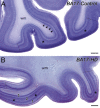
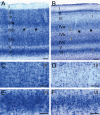
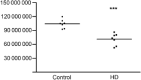
Huntington's disease (HD), an autosomal dominantly inherited polyglutamine or CAG repeat disease along with somatomotor, oculomotor, psychiatric and cognitive symptoms, presents clinically with impairments of elementary and complex visual functions as well as altered visual-evoked potentials (VEPs). Previous volumetric and pathoanatomical post-mortem investigations pointed to an involvement of Brodmann's primary visual area 17 (BA17) in HD. Because the involvement of BA17 could be interpreted as an early onset brain neurodegeneration, we further characterized this potential primary cortical site of HD-related neurodegeneration neuropathologically and performed an unbiased estimation of the absolute nerve cell number in thick gallocyanin-stained frontoparallel tissue sections through the striate area of seven control individuals and seven HD patients using Cavalieri's principle for volume and the optical disector for nerve and glial cell density estimations. This investigation showed a reduction of the estimated absolute nerve cell number of BA17 in the HD patients (71,044,037 ± 12,740,515 nerve cells) of 32% in comparison with the control individuals (104,075,067 ± 9,424,491 nerve cells) (Mann-Whitney U-test; P < 0.001). Additional pathoanatomical studies showed that nerve cell loss was most prominent in the outer pyramidal layer III, the inner granular layers IVa and IVc as well as in the multiform layer VI of BA17 of the HD patients. Our neuropathological results in BA17 confirm and extend previous post-mortem, biochemical and in vivo neuroradiological HD findings and offer suitable explanations for the elementary and complex visual dysfunctions, as well as for the altered VEP observed in HD patients.
Keywords: Huntington's disease; area 17; cerebral cortex; polyglutamine diseases; visual system; visual-evoked potentials (VEP).
© 2014 International Society of Neuropathology.
Figures
References
-
- Akil M, Lewis DA (1993) The dopaminergic innervation of monkey entorhinal cortex. Cereb Cortex 3:533–550. - PubMed
-
- Alheid GF, Heimer L, Switzer RC (1990) Basal ganglia. In: The Human Nervous System. Paxinos G (ed.), pp. 483–582. Academic Press: San Diego.
-
- Braak H, Braak E (1992) Allocortical involvement in Huntington's disease. Neuropathol Appl Neurobiol 18:539–547. - PubMed
-
- Brodmann K (1909) Vergleichende Lokalisationslehre der Großhirnrinde. Johann Ambrosius Barth: Leipzig.
-
- Brouwers P, Cox C, Martin A, Chase T, Fedio P (1984) Differential perceptual‐spatial impairment in Huntington's and Alzheimer's dementias. Arch Neurol 41:1073–1076. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

