Adjuvant ovarian suppression in premenopausal breast cancer
- PMID: 25495490
- PMCID: PMC4341825
- DOI: 10.1056/NEJMoa1412379
Adjuvant ovarian suppression in premenopausal breast cancer
Abstract
Background: Suppression of ovarian estrogen production reduces the recurrence of hormone-receptor-positive early breast cancer in premenopausal women, but its value when added to tamoxifen is uncertain.
Methods: We randomly assigned 3066 premenopausal women, stratified according to prior receipt or nonreceipt of chemotherapy, to receive 5 years of tamoxifen, tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. The primary analysis tested the hypothesis that tamoxifen plus ovarian suppression would improve disease-free survival, as compared with tamoxifen alone. In the primary analysis, 46.7% of the patients had not received chemotherapy previously, and 53.3% had received chemotherapy and remained premenopausal.
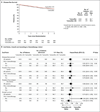
Results: After a median follow-up of 67 months, the estimated disease-free survival rate at 5 years was 86.6% in the tamoxifen-ovarian suppression group and 84.7% in the tamoxifen group (hazard ratio for disease recurrence, second invasive cancer, or death, 0.83; 95% confidence interval [CI], 0.66 to 1.04; P=0.10). Multivariable allowance for prognostic factors suggested a greater treatment effect with tamoxifen plus ovarian suppression than with tamoxifen alone (hazard ratio, 0.78; 95% CI, 0.62 to 0.98). Most recurrences occurred in patients who had received prior chemotherapy, among whom the rate of freedom from breast cancer at 5 years was 82.5% in the tamoxifen-ovarian suppression group and 78.0% in the tamoxifen group (hazard ratio for recurrence, 0.78; 95% CI, 0.60 to 1.02). At 5 years, the rate of freedom from breast cancer was 85.7% in the exemestane-ovarian suppression group (hazard ratio for recurrence vs. tamoxifen, 0.65; 95% CI, 0.49 to 0.87).
Conclusions: Adding ovarian suppression to tamoxifen did not provide a significant benefit in the overall study population. However, for women who were at sufficient risk for recurrence to warrant adjuvant chemotherapy and who remained premenopausal, the addition of ovarian suppression improved disease outcomes. Further improvement was seen with the use of exemestane plus ovarian suppression. (Funded by Pfizer and others; SOFT ClinicalTrials.gov number, NCT00066690.).
Figures


Comment in
-
Perfecting breast-cancer treatment--incremental gains and musculoskeletal pains.N Engl J Med. 2015 Jan 29;372(5):477-8. doi: 10.1056/NEJMe1413164. Epub 2014 Dec 11. N Engl J Med. 2015. PMID: 25495491 No abstract available.
-
Adjuvant ovarian suppression in premenopausal breast cancer.N Engl J Med. 2015 Apr 23;372(17):1673. doi: 10.1056/NEJMc1502618. N Engl J Med. 2015. PMID: 25901437 No abstract available.
-
Adjuvant ovarian suppression in premenopausal breast cancer.N Engl J Med. 2015 Apr 23;372(17):1672-3. doi: 10.1056/NEJMc1502618. N Engl J Med. 2015. PMID: 25901438 No abstract available.
-
SOFT trial can be very hard on young women.Breast. 2015 Dec;24(6):767-8. doi: 10.1016/j.breast.2015.08.010. Epub 2015 Sep 19. Breast. 2015. PMID: 26387600 No abstract available.
References
-
- Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst. 2001;93:979–989. - PubMed
-
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Senn HJ. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. J Clin Oncol. 2001;19:3817–3827. - PubMed
-
- LHRH-Agonists in Early Breast Cancer Overview Group. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711–1723. - PubMed
-
- Griggs JJ, Somerfield MR, Anderson H, et al. American Society of Clinical Oncology endorsement of the Cancer Care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol. 2011;29:3939–3942. [Erratum, J Clin Oncol 2012; 30: 1398.] - PubMed
-
- Pagani O, O’Neill A, Castiglione M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–640. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
