Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury
- PMID: 25495836
- PMCID: PMC6495224
- DOI: 10.1111/cns.12362
Inhibition of G protein-coupled receptor 81 (GPR81) protects against ischemic brain injury
Abstract
Aim: Lactates accumulate in ischemic brains. G protein-coupled receptor 81 (GPR81) is an endogenous receptor for lactate. We aimed to explore whether lactate is involved in ischemic injury via activating GPR81.
Methods: N2A cells were transfected with GFP-GPR81 plasmids 24 h previously, and then treated with GPR81 antagonist 3-hydroxy-butyrate (3-OBA) alone or cotreated with agonists lactate or 3, 5-dihydroxybenzoic acid (3, 5-DHBA) during 3 h of oxygen-glucose deprivation (OGD). Adult male C57BL/6J mice and primary cultured cortical neurons were treated with 3-OBA at the onset of middle cerebral artery occlusion (MCAO) or OGD, respectively.
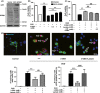
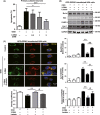
Results: The GPR81 overexpression increased the cell vulnerability to ischemic injury. And GPR81 antagonism by 3-OBA significantly prevented cell death and brain injury after OGD and MCAO, respectively. Furthermore, inhibition of GPR81 reversed ischemia-induced apoptosis and extracellular signal-regulated kinase (ERK) signaling may be involved in the neuroprotection.
Conclusions: G protein-coupled receptor 81 (GPR81) inhibition attenuated ischemic neuronal death. Lactate may aggravate ischemic brain injury by activating GPR81. GPR81 antagonism might be a novel therapeutic strategy for the treatment of cerebral ischemia.
Keywords: Cerebral ischemia; G protein-coupled receptor 81; Lactates; Neuroprotection.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Lactate Protects Microglia and Neurons from Oxygen-Glucose Deprivation/Reoxygenation.Neurochem Res. 2024 Jul;49(7):1762-1781. doi: 10.1007/s11064-024-04135-7. Epub 2024 Mar 29. Neurochem Res. 2024. PMID: 38551797
-
Dual Properties of Lactate in Müller Cells: The Effect of GPR81 Activation.Invest Ophthalmol Vis Sci. 2019 Mar 1;60(4):999-1008. doi: 10.1167/iovs.18-25458. Invest Ophthalmol Vis Sci. 2019. PMID: 30884529
-
Downregulation of miRNA-134 protects neural cells against ischemic injury in N2A cells and mouse brain with ischemic stroke by targeting HSPA12B.Neuroscience. 2014 Sep 26;277:111-22. doi: 10.1016/j.neuroscience.2014.06.062. Epub 2014 Jul 5. Neuroscience. 2014. PMID: 25003713
-
History and Function of the Lactate Receptor GPR81/HCAR1 in the Brain: A Putative Therapeutic Target for the Treatment of Cerebral Ischemia.Neuroscience. 2023 Aug 21;526:144-163. doi: 10.1016/j.neuroscience.2023.06.022. Epub 2023 Jun 29. Neuroscience. 2023. PMID: 37391123 Review.
-
Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon.Pharmacol Ther. 2020 Feb;206:107451. doi: 10.1016/j.pharmthera.2019.107451. Epub 2019 Dec 10. Pharmacol Ther. 2020. PMID: 31836453 Review.
Cited by
-
Lactate Metabolism and Signaling in Tuberculosis and Cancer: A Comparative Review.Front Cell Infect Microbiol. 2021 Feb 26;11:624607. doi: 10.3389/fcimb.2021.624607. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33718271 Free PMC article. Review.
-
Lactate metabolism in human health and disease.Signal Transduct Target Ther. 2022 Sep 1;7(1):305. doi: 10.1038/s41392-022-01151-3. Signal Transduct Target Ther. 2022. PMID: 36050306 Free PMC article. Review.
-
Dual Blockade of Lactate/GPR81 and PD-1/PD-L1 Pathways Enhances the Anti-Tumor Effects of Metformin.Biomolecules. 2021 Sep 17;11(9):1373. doi: 10.3390/biom11091373. Biomolecules. 2021. PMID: 34572586 Free PMC article.
-
Lactate Metabolism and Lactylation Modification: New Opportunities and Challenges in Cardiovascular Disease.MedComm (2020). 2025 Jul 1;6(7):e70269. doi: 10.1002/mco2.70269. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599233 Free PMC article. Review.
-
Lactylation in cancer: Mechanisms in tumour biology and therapeutic potentials.Clin Transl Med. 2024 Nov;14(11):e70070. doi: 10.1002/ctm2.70070. Clin Transl Med. 2024. PMID: 39456119 Free PMC article. Review.
References
-
- Combs DJ, Dempsey RJ, Maley M, Donaldson D, Smith C. Relationship between plasma glucose, brain lactate, and intracellular ph during cerebral ischemia in gerbils. Stroke 1990;21:936–942. - PubMed
-
- Liu C, Wu J, Zhu J, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan g‐protein‐coupled receptor, gpr81. J Biol Chem 2009;284:2811–2822. - PubMed
-
- Cai TQ, Ren N, Jin L, et al. Role of gpr81 in lactate‐mediated reduction of adipose lipolysis. Biochem Biophys Res Commun 2008;377:987–991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

