A population-based cohort study on adherence to practice guidelines for adjuvant chemotherapy in colorectal cancer
- PMID: 25495897
- PMCID: PMC4301907
- DOI: 10.1186/1471-2407-14-948
A population-based cohort study on adherence to practice guidelines for adjuvant chemotherapy in colorectal cancer
Abstract
Background: The value of adjuvant chemotherapy in colorectal cancer is well studied, and guidelines have been established. Little is known about how treatment guidelines are implemented in the everyday clinical setting.
Methods: This national population-based study on nearly 34,000 patients with colorectal cancer evaluates the adherence to present clinical guidelines for adjuvant chemotherapy. Virtually all patients with colorectal cancer in Sweden during the years 2007-2012 and data from the Swedish Colorectal Cancer Registry were included.
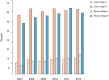
Results: In colon cancer stage III, adherence to national guidelines was associated with lower age, presence of multidisciplinary team (MDT) conference, low co-morbidity, and worse N stage. The MDT forum also affected whether or not high-risk stage II colon cancer patients were considered for adjuvant chemotherapy. Rectal cancer patients both in stage II and III were considered for adjuvant chemotherapy less often than colon cancer patients, but the same factors influenced the decision. Adjuvant chemotherapy was started later than eight weeks after surgery in 30% of colon cancer patients and in 38% of rectal cancer patients.
Conclusions: In Sweden, the adherence to national guidelines for adjuvant chemotherapy in colon cancer stage III is acceptable in younger and healthier patients. MDT conferences are of major importance and affect whether patients are recommended for adjuvant chemotherapy. Special consideration needs to be given to certain subgroups of patients, particularly older patients and patients with poorly differentiated tumors. There is a need to shorten the waiting time until start of chemotherapy.
Figures
References
-
- Kolorektal cancer: Nationellt vårdprogram: 2008 (in English: Colorectal cancer: National treatment plan: 2008) 2008.http://www.cancercentrum.se/PageFiles/2823/Kolorektal%20cancer%20nya_var...
-
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J, Nagtegaal ID, Beets-Tan RG, Arnold D, Ciardiello F, Hoff P, Kerr D, Kohne CH, Labianca R, Price T, Scheithauer W, Sobrero A, Tabernero J, Aderka D, Barroso S, Bodoky G, Douillard JY, El Ghazaly H, Gallardo J, Garin A, Glynne-Jones R, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. - DOI - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2407/14/948/prepub
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

