Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization
- PMID: 25496128
- PMCID: PMC4295566
- DOI: 10.1186/1476-4598-13-264
Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization
Abstract
Background: Na(V)1.5 voltage-gated sodium channels are abnormally expressed in breast tumours and their expression level is associated with metastatic occurrence and patients' death. In breast cancer cells, Na(V)1.5 activity promotes the proteolytic degradation of the extracellular matrix and enhances cell invasiveness.
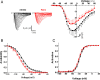
Findings: In this study, we showed that the extinction of Na(V)1.5 expression in human breast cancer cells almost completely abrogated lung colonisation in immunodepressed mice (NMRI nude). Furthermore, we demonstrated that ranolazine (50 μM) inhibited Na(V)1.5 currents in breast cancer cells and reduced Na(V)1.5-related cancer cell invasiveness in vitro. In vivo, the injection of ranolazine (50 mg/kg/day) significantly reduced lung colonisation by Na(V)1.5-expressing human breast cancer cells.
Conclusions: Taken together, our results demonstrate the importance of Na(V)1.5 in the metastatic colonisation of organs by breast cancer cells and indicate that small molecules interfering with Na(V) activity, such as ranolazine, may represent powerful pharmacological tools to inhibit metastatic development and improve cancer treatments.
Figures


References
-
- Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, Lei M, Bougnoux P, Gruel Y, Le Guennec JY. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39:774–786. doi: 10.1016/j.biocel.2006.12.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

