Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study
- PMID: 25496464
- PMCID: PMC4819568
- DOI: 10.3109/14397595.2014.995875
Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study
Abstract
Objectives: To evaluate oral tofacitinib versus placebo for treatment of active rheumatoid arthritis in Japanese patients with inadequate response to disease-modifying antirheumatic drugs.
Methods: In this double-blind, placebo-controlled, randomized, parallel-group, 12-week, phase 2 study (clinicaltrials.gov NCT00687193), 317 patients received tofacitinib: 1, 3, 5, 10, or 15 mg as monotherapy or placebo twice daily (BID).
Primary endpoint: response rate by American College of Rheumatology (ACR) ≥ 20% improvement criteria (ACR20) at week 12.
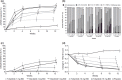
Results: ACR20 response rates: 37.7% (20/53), 67.9% (36/53), 73.1% (38/52), 84.9% (45/53), and 90.7% (49/54) with tofacitinib: 1, 3, 5, 10, and 15 mg BID, respectively, versus 15.4% (8/52) with placebo (p < 0.01; all doses). Dose-dependent ACR20 responses with tofacitinib versus placebo occurred from week 2 onward (p < 0.05). Changes from baseline in 28-joint disease activity score using erythrocyte sedimentation rate improved with tofacitinib versus placebo from week 4 (p < 0.01; all doses). Six tofacitinib patients experienced treatment-related serious adverse events (AEs). Most common treatment-emergent AEs: nasopharyngitis (10% vs 12%) and hyperlipidemia (5% vs 0%). Serum creatinine, hemoglobin, and total-, low-, and high-density lipoprotein-cholesterol levels increased with tofacitinib.
Conclusions: Tofacitinib produced dose-dependent ACR20 responses and reduced disease activity. The safety profile was consistent with that reported from global monotherapy trials.
Keywords: Japan; Monotherapy; Randomized controlled trial; Rheumatoid arthritis; Tofacitinib.
Figures
References
-
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59((6)):762–84. - PubMed
-
- Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;13((Suppl 9)):S237–51. - PubMed
-
- Koike R, Takeuchi T, Eguchi K, Miyasaka N. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod Rheumatol. 2007;17((6)):451–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

