Rfx6 maintains the functional identity of adult pancreatic β cells
- PMID: 25497096
- PMCID: PMC4542305
- DOI: 10.1016/j.celrep.2014.11.033
Rfx6 maintains the functional identity of adult pancreatic β cells
Abstract
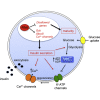
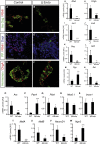
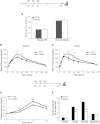
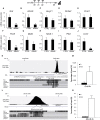
Increasing evidence suggests that loss of β cell characteristics may cause insulin secretory deficiency in diabetes, but the underlying mechanisms remain unclear. Here, we show that Rfx6, whose mutation leads to neonatal diabetes in humans, is essential to maintain key features of functionally mature β cells in mice. Rfx6 loss in adult β cells leads to glucose intolerance, impaired β cell glucose sensing, and defective insulin secretion. This is associated with reduced expression of core components of the insulin secretion pathway, including glucokinase, the Abcc8/SUR1 subunit of KATP channels and voltage-gated Ca(2+) channels, which are direct targets of Rfx6. Moreover, Rfx6 contributes to the silencing of the vast majority of "disallowed" genes, a group usually specifically repressed in adult β cells, and thus to the maintenance of β cell maturity. These findings raise the possibility that changes in Rfx6 expression or activity may contribute to β cell failure in humans.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ainscow E.K., Zhao C., Rutter G.A. Acute overexpression of lactate dehydrogenase-A perturbs beta-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. - PubMed
-
- Artuso R., Provenzano A., Mazzinghi B., Giunti L., Palazzo V., Andreucci E., Blasetti A., Chiuri R.M., Gianiorio F.E., Mandich P. Therapeutic implications of novel mutations of the RFX6 gene associated with early-onset diabetes. Pharmacogenomics J. 2014 Published online July 22, 2014. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

