Floral biology and ovule and seed ontogeny of Nymphaea thermarum, a water lily at the brink of extinction with potential as a model system for basal angiosperms
- PMID: 25497514
- PMCID: PMC4551091
- DOI: 10.1093/aob/mcu235
Floral biology and ovule and seed ontogeny of Nymphaea thermarum, a water lily at the brink of extinction with potential as a model system for basal angiosperms
Abstract
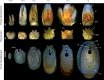
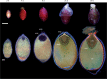
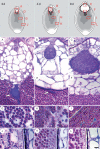
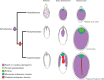
Background and aims: Nymphaea thermarum is a member of the Nymphaeales, of one of the most ancient lineages of flowering plants. This species was only recently described and then declared extinct in the wild, so little is known about its reproductive biology. In general, the complete ontogeny of ovules and seeds is not well documented among species of Nymphaea and has never been studied in the subgenus Brachyceras, the clade to which N. thermarum belongs.
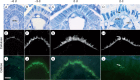
Methods: Flowers and fruits were processed for brightfield, epifluorescence and confocal microscopy. Flower morphology, with emphasis on the timing of male and female functions, was correlated with key developmental stages of the ovule and the female gametophyte. Development of the seed tissues and dynamics of polysaccharide reserves in the endosperm, perisperm and embryo were examined.
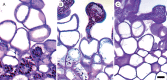
Key results: Pollen release in N. thermarum starts before the flower opens. Cell walls of the micropylar nucellus show layering of callose and cellulose in a manner reminiscent of transfer cell wall patterning. Endosperm development is ab initio cellular, with micropylar and chalazal domains that embark on distinct developmental trajectories. The surrounding maternal perisperm occupies the majority of seed volume and accumulates starch centrifugally. In mature seeds, a minute but fully developed embryo is surrounded by a single, persistent layer of endosperm.
Conclusions: Early male and female function indicate that N. thermarum is predisposed towards self-pollination, a phenomenon that is likely to have evolved multiple times within Nymphaea. While formation of distinct micropylar and chalazal developmental domains in the endosperm, along with a copious perisperm, characterize the seeds of most members of the Nymphaeales, seed ontogenies vary between and among the constituent families. Floral biology, life history traits and small genome size make N. thermarum uniquely promising as an early-diverging angiosperm model system for genetic and molecular studies.
Keywords: Early-diverging angiosperm; Nymphaea thermarum; Nymphaeales; embryo; endosperm; evo-devo; female gametophyte; flower biology; megagametogenesis; megasporogenesis; perisperm; protogyny; seed development; stigma.
© The Author 2014. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Baker HG, Baker I. 1979. Starch in angiosperm pollen grains and its evolutionary significance. American Journal of Botany 66: 591–600.
-
- Barrell P, Grossniklaus U. 2005. Confocal microscopy of whole ovules for analysis of reproductive development: the elongate1 mutant affects meiosis II. Plant Journal 43: 309–320. - PubMed
-
- Baskin CC, Baskin JM. 2007. Nymphaeaceae: a basal angiosperm family (ANITA grade) with a fully developed embryo. Seed Science Research 17: 293–296.
-
- Batygina TB, Kravtsova TI, Shamrov II. 1980. The comparative embryology of some representatives of the orders Nymphaeales and Nelumbonales. Botanicheskii Zhurnal 65: 1071–1086.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

