Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways
- PMID: 25497688
- PMCID: PMC4459946
- DOI: 10.1016/j.nbd.2014.11.018
Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways
Abstract
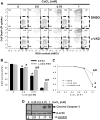
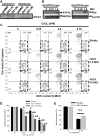
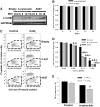
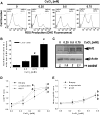
Gene multiplications or point mutations in alpha (α)-synuclein are associated with familial and sporadic Parkinson's disease (PD). An increase in copper (Cu) levels has been reported in the cerebrospinal fluid and blood of PD patients, while occupational exposure to Cu has been suggested to augment the risk to develop PD. We aimed to elucidate the mechanisms by which α-synuclein and Cu regulate dopaminergic cell death. Short-term overexpression of wild type (WT) or mutant A53T α-synuclein had no toxic effect in human dopaminergic cells and primary midbrain cultures, but it exerted a synergistic effect on Cu-induced cell death. Cell death induced by Cu was potentiated by overexpression of the Cu transporter protein 1 (Ctr1) and depletion of intracellular glutathione (GSH) indicating that the toxic effects of Cu are linked to alterations in its intracellular homeostasis. Using the redox sensor roGFP, we demonstrated that Cu-induced oxidative stress was primarily localized in the cytosol and not in the mitochondria. However, α-synuclein overexpression had no effect on Cu-induced oxidative stress. WT or A53T α-synuclein overexpression exacerbated Cu toxicity in dopaminergic and yeast cells in the absence of α-synuclein aggregation. Cu increased autophagic flux and protein ubiquitination. Impairment of autophagy by overexpression of a dominant negative Atg5 form or inhibition of the ubiquitin/proteasome system (UPS) with MG132 enhanced Cu-induced cell death. However, only inhibition of the UPS stimulated the synergistic toxic effects of Cu and α-synuclein overexpression. Our results demonstrate that α-synuclein stimulates Cu toxicity in dopaminergic cells independent from its aggregation via modulation of protein degradation pathways.
Keywords: A53T; AMPK; Autophagy; Copper and Parkinson's disease; Oxidative stress; Proteasome; Ubiquitination; α-Synuclein.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors certify that there is no conflict of interest.
Figures
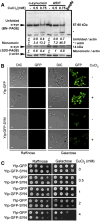
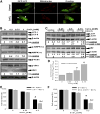
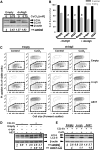

References
-
- Alvarez-Castelao B, et al. Mechanism of cleavage of alpha-synuclein by the 20S proteasome and modulation of its degradation by the RedOx state of the N-terminal methionines. Biochim Biophys Acta 2013 - PubMed
-
- Arnal N, et al. Clinical utility of copper, ceruloplasmin, and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res. 2010;1319:118–130. - PubMed
-
- Auluck PK, et al. alpha-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–233. - PubMed
-
- Ayton S, et al. Ceruloplasmin dysfunction and therapeutic potential for parkinson disease. Ann Neurol 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

