Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial
- PMID: 25497694
- PMCID: PMC4772450
- DOI: 10.1016/S1470-2045(14)71171-4
Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial
Abstract
Background: Four previously published randomised clinical trials have shown that tamoxifen can reduce the risk of breast cancer in healthy women at increased risk of breast cancer in the first 10 years of follow-up. We report the long-term follow-up of the IBIS-I trial, in which the participants and investigators remain largely masked to treatment allocation.
Methods: In the IBIS-I randomised controlled trial, premenopausal and postmenopausal women 35-70 years of age deemed to be at an increased risk of developing breast cancer were randomly assigned (1:1) to receive oral tamoxifen 20 mg daily or matching placebo for 5 years. Patients were randomly assigned to the two treatment groups by telephone or fax according to a block randomisation schedule (permuted block sizes of six or ten). Patients and investigators were masked to treatment assignment by use of central randomisation and coded drug supply. The primary endpoint was the occurrence of breast cancer (invasive breast cancer and ductal carcinoma in situ), analysed by intention to treat. Cox proportional hazard models were used to assess breast cancer occurrence and mortality. The trial is closed to recruitment and active treatment is completed, but long-term follow-up is ongoing. This trial is registered with controlledtrials.com, number ISRCTN91879928.
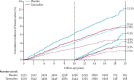
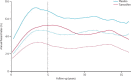
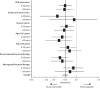
Findings: Between April 14, 1992, and March 30, 2001, 7154 eligible women recruited from genetics clinics and breast care clinics in eight countries were enrolled into the IBIS-I trial and were randomly allocated to the two treatment groups: 3579 to tamoxifen and 3575 to placebo. After a median follow up of 16.0 years (IQR 14.1-17.6), 601 breast cancers have been reported (251 [7.0%] in 3579 patients in the tamoxifen group vs 350 [9.8%] in 3575 women in the placebo group; hazard ratio [HR] 0.71 [95% CI 0.60-0.83], p<0.0001). The risk of developing breast cancer was similar between years 0-10 (226 [6.3%] in 3575 women in the placebo group vs 163 [4.6%] in 3579 women in the tamoxifen group; hazard ratio [HR] 0.72 [95% CI 0.59-0.88], p=0.001) and after 10 years (124 [3.8%] in 3295 women vs 88 [2.6%] in 3343, respectively; HR 0.69 [0.53-0.91], p=0.009). The greatest reduction in risk was seen in invasive oestrogen receptor-positive breast cancer (HR 0.66 [95% CI 0.54-0.81], p<0.0001) and ductal carcinoma in situ (0.65 [0.43-1.00], p=0.05), but no effect was noted for invasive oestrogen receptor-negative breast cancer (HR 1.05 [95% CI 0.71-1.57], p=0.8).
Interpretation: These results show that tamoxifen offers a very long period of protection after treatment cessation, and thus substantially improves the benefit-to-harm ratio of the drug for breast cancer prevention.
Funding: Cancer Research UK (UK) and the National Health and Medical Research Council (Australia).
Copyright © 2015 Cuzick et al. Open Access article distributed under the terms of CC BY. Published by Elsevier Ltd. All rights reserved.
Figures
Comment in
-
IBIS-I tamoxifen update: maturity brings questions.Lancet Oncol. 2015 Jan;16(1):7-9. doi: 10.1016/S1470-2045(14)71184-2. Epub 2014 Dec 11. Lancet Oncol. 2015. PMID: 25497695 No abstract available.
-
Tamoxifen reduces breast cancer rate in at-risk healthy women by nearly a third, finds study.BMJ. 2014 Dec 11;349:g7596. doi: 10.1136/bmj.g7596. BMJ. 2014. PMID: 25500812 No abstract available.
-
Breast cancer: tamoxifen - offering a long-term prevention option.Nat Rev Clin Oncol. 2015 Feb;12(2):66. doi: 10.1038/nrclinonc.2014.236. Epub 2015 Jan 13. Nat Rev Clin Oncol. 2015. PMID: 25583681 No abstract available.
-
ACP Journal Club. Tamoxifen for 5 years reduced 16-year risk for breast cancer in women at increased risk.Ann Intern Med. 2015 May 19;162(10):JC11. doi: 10.7326/ACPJC-2015-162-10-011. Ann Intern Med. 2015. PMID: 25984872 No abstract available.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2:282. - PubMed
-
- Fisher B, Costantino JP, Wickerham DL. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. - PubMed
-
- Fisher B, Costantino JP, Wickerham DL. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. - PubMed
-
- Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

