Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP)
- PMID: 25497733
- PMCID: PMC4323933
- DOI: 10.1016/j.nbd.2014.12.001
Phenotypic characterization of a Csf1r haploinsufficient mouse model of adult-onset leukodystrophy with axonal spheroids and pigmented glia (ALSP)
Abstract
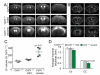
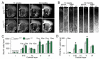
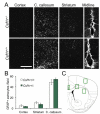
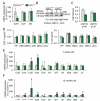
Mutations in the colony stimulating factor-1 receptor (CSF1R) that abrogate the expression of the affected allele or lead to the expression of mutant receptor chains devoid of kinase activity have been identified in both familial and sporadic cases of ALSP. To determine the validity of the Csf1r heterozygous mouse as a model of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) we performed behavioral, radiologic, histopathologic, ultrastructural and cytokine expression studies of young and old Csf1r+/- and control Csf1r+/+ mice. Six to 8-month old Csf1r+/- mice exhibit cognitive deficits, and by 9-11 months develop sensorimotor deficits and in male mice, depression and anxiety-like behavior. MRIs of one year-old Csf1r+/- mice reveal lateral ventricle enlargement and thinning of the corpus callosum. Ultrastructural analysis of the corpus callosum uncovers dysmyelinated axons as well as neurodegeneration, evidenced by the presence of axonal spheroids. Histopathological examination of 11-week-old mice reveals increased axonal and myelin staining in the cortex, increase of neuronal cell density in layer V and increase of microglial cell densities throughout the brain, suggesting that early developmental changes contribute to disease. By 10-months of age, the neuronal cell density normalizes, oligodendrocyte precursor cells increase in layers II-III and V and microglial densities remain elevated without an increase in astrocytes. Also, the age-dependent increase in CSF-1R+ neurons in cortical layer V is reduced. Moreover, the expression of Csf2, Csf3, Il27 and Il6 family cytokines is increased, consistent with microglia-mediated inflammation. These results demonstrate that the inactivation of one Csf1r allele is sufficient to cause an ALSP-like disease in mice. The Csf1r+/- mouse is a model of ALSP that will allow the critical events for disease development to be determined and permit rapid evaluation of therapeutic approaches. Furthermore, our results suggest that aberrant activation of microglia in Csf1r+/- mice may play a central role in ALSP pathology.
Keywords: ALSP; CSF-1R; Dysmyelination; GM-CSF; HDLS; Leukodystrophy; Microglia.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





References
-
- Axelsson R, et al. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. - PubMed
-
- Baldwin GC, et al. Identification and characterization of a high-affinity granulocyte-macrophage colony-stimulating factor receptor on primary rat oligodendrocytes. Blood. 1993;82:3279–82. - PubMed
-
- Caterini F, et al. Object recognition and object orientation in Alzheimer's disease. Neuropsychology. 2002;16:146–55. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

