Mouse ERG K(+) channel clones reveal differences in protein trafficking and function
- PMID: 25497881
- PMCID: PMC4338741
- DOI: 10.1161/JAHA.114.001491
Mouse ERG K(+) channel clones reveal differences in protein trafficking and function
Abstract
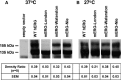
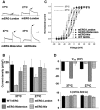
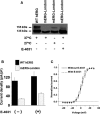
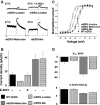
Background: The mouse ether-a-go-go-related gene 1a (mERG1a, mKCNH2) encodes mERG K(+) channels in mouse cardiomyocytes. The mERG channels and their human analogue, hERG channels, conduct IKr. Mutations in hERG channels reduce IKr to cause congenital long-QT syndrome type 2, mostly by decreasing surface membrane expression of trafficking-deficient channels. Three cDNA sequences were originally reported for mERG channels that differ by 1 to 4 amino acid residues (mERG-London, mERG-Waterston, and mERG-Nie). We characterized these mERG channels to test the postulation that they would differ in their protein trafficking and biophysical function, based on previous findings in long-QT syndrome type 2.
Methods and results: The 3 mERG and hERG channels were expressed in HEK293 cells and neonatal mouse cardiomyocytes and were studied using Western blot and whole-cell patch clamp. We then compared our findings with the recent sequencing results in the Welcome Trust Sanger Institute Mouse Genomes Project (WTSIMGP).
Conclusions: First, the mERG-London channel with amino acid substitutions in regions of highly ordered structure is trafficking deficient and undergoes temperature-dependent and pharmacological correction of its trafficking deficiency. Second, the voltage dependence of channel gating would be different for the 3 mERG channels. Third, compared with the WTSIMGP data set, the mERG-Nie clone is likely to represent the wild-type mouse sequence and physiology. Fourth, the WTSIMGP analysis suggests that substrain-specific sequence differences in mERG are a common finding in mice. These findings with mERG channels support previous findings with hERG channel structure-function analyses in long-QT syndrome type 2, in which sequence changes in regions of highly ordered structure are likely to result in abnormal protein trafficking.
Keywords: genetic variability; hERG; long‐QT syndrome; mERG; mouse.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995; 81:299-307. - PubMed
-
- Trudeau MC, Warmke JW, Ganezky B, Robertson GA. HERG, a human inward rectifier in the voltage‐gated potassium channel family. Science. 1995; 269:92-95. - PubMed
-
- London B, Trudeau MC, Newton KP, Beyer AK, Copeland NG, Gilbert DJ, Jenkins NA, Satler CA, Robertson GA. Two isoforms of the mouse ether‐a‐go‐go‐related gene coassemble to form channels with properties similar to the rapidly activating component of the cardiac delayed rectifier K+ current. Circ Res. 1997; 81:870-878. - PubMed
-
- Zhou Z, Gong Q, Epstein M, January CT. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem. 1998; 273:21061-21066. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

