Downregulation of TES by hypermethylation in glioblastoma reduces cell apoptosis and predicts poor clinical outcome
- PMID: 25498217
- PMCID: PMC4279594
- DOI: 10.1186/s40001-014-0066-4
Downregulation of TES by hypermethylation in glioblastoma reduces cell apoptosis and predicts poor clinical outcome
Abstract
Background: Gliomas are the most common human brain tumors. Glioblastoma, also known as glioblastoma multiform (GBM), is the most aggressive, malignant, and lethal glioma. The investigation of prognostic and diagnostic molecular biomarkers in glioma patients to provide direction on clinical practice is urgent. Recent studies demonstrated that abnormal DNA methylation states play a key role in the pathogenesis of this kind of tumor. In this study, we want to identify a novel biomarker related to glioma initiation and find the role of the glioma-related gene.
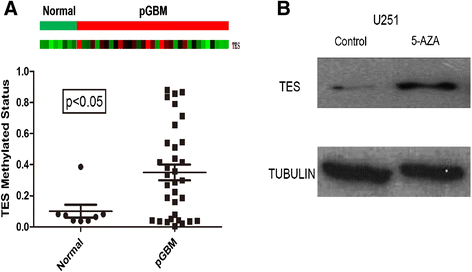
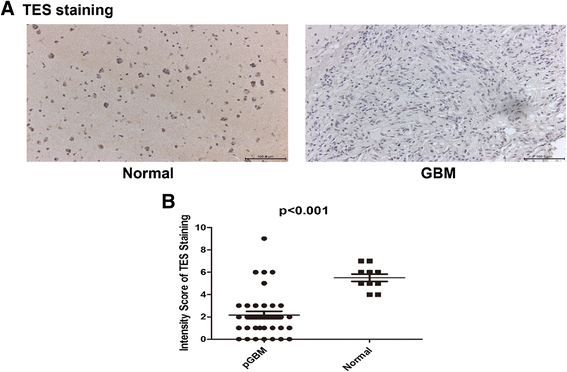
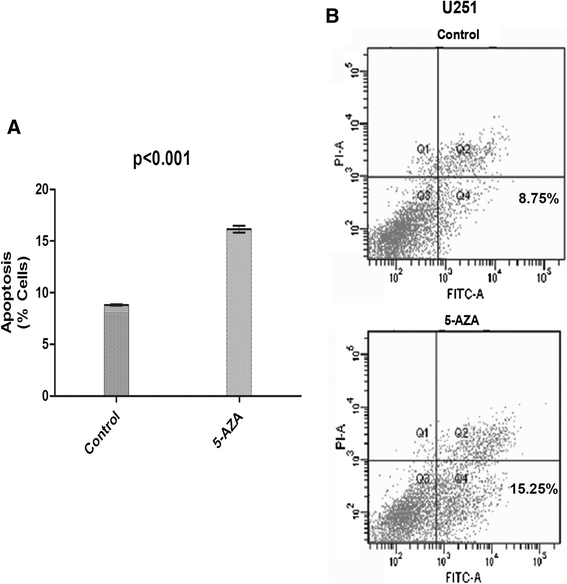
Methods: We performed a methylation-specific microarray on the promoter region to identify methylation gene(s) that may affect outcome of GBM patients. Normal and GBM tissues were collected from Tiantan Hospital. Genomic DNA was extracted from these tissues and analyzed with a DNA promoter methylation microarray. Testis derived transcript (TES) protein expression was analyzed by immunohistochemistry in paraffin-embedded patient tissues. Western blotting was used to detect TES protein expression in the GBM cell line U251 with or without 5-aza-dC treatment. Cell apoptosis was evaluated by flow cytometry analysis using Annexin V/PI staining.
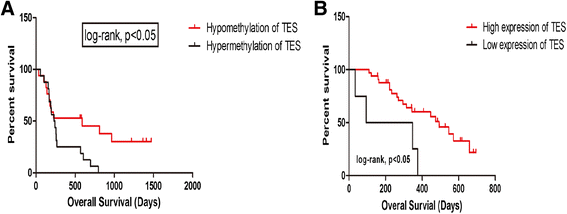
Results: We found that the TES promoter was hypermethylated in GBM compared to normal brain tissues under DNA promoter methylation microarray analysis. The GBM patients with TES hypermethylation had a short overall survival (P <0.05, log-rank test). Among GBM samples, reduced TES protein level was detected in 33 (89.2%) of 37 tumor tissues by immunohistochemical staining. Down regulation of TES was also correlated with worse patient outcome (P <0.05, log-rank test). Treatment on the GBM cell line U251 with 5-aza-dC can greatly increase TES expression, confirming the hypermethylation of TES promoter in GBM. Up-regulation of TES prompts U251 apoptosis significantly. This study demonstrated that both TES promoter hypermethylation and down-regulated protein expression significantly correlated with worse patient outcome. Treatment on the GBM cell line (U251) with 5-aza-dC can highly release TES expression resulting in significant apoptosis in these cells.
Conclusions: Our findings suggest that the TES gene is a novel tumor suppressor gene and might represent a valuable prognostic marker for glioblastoma, indicating a potential target for future GBM therapy.
Figures
References
-
- Christensen BC, Smith AA, Zheng S, Koestler DC, Houseman EA, Marsit CJ, Wiemels JL, Nelson HH, Karagas MR, Wrensch MR, Kelsey KT, Wiencke JK. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103:143–153. doi: 10.1093/jnci/djq497. - DOI - PMC - PubMed
-
- Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RG, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K, Cancer Genome Atlas Research Network Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

