The impact of gradient strength on in vivo diffusion MRI estimates of axon diameter
- PMID: 25498429
- PMCID: PMC4285777
- DOI: 10.1016/j.neuroimage.2014.12.008
The impact of gradient strength on in vivo diffusion MRI estimates of axon diameter
Abstract
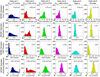
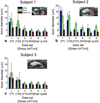
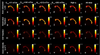
Diffusion magnetic resonance imaging (MRI) methods for axon diameter mapping benefit from higher maximum gradient strengths than are currently available on commercial human scanners. Using a dedicated high-gradient 3T human MRI scanner with a maximum gradient strength of 300 mT/m, we systematically studied the effect of gradient strength on in vivo axon diameter and density estimates in the human corpus callosum. Pulsed gradient spin echo experiments were performed in a single scan session lasting approximately 2h on each of three human subjects. The data were then divided into subsets with maximum gradient strengths of 77, 145, 212, and 293 mT/m and diffusion times encompassing short (16 and 25 ms) and long (60 and 94 ms) diffusion time regimes. A three-compartment model of intra-axonal diffusion, extra-axonal diffusion, and free diffusion in cerebrospinal fluid was fitted to the data using a Markov chain Monte Carlo approach. For the acquisition parameters, model, and fitting routine used in our study, it was found that higher maximum gradient strengths decreased the mean axon diameter estimates by two to three fold and decreased the uncertainty in axon diameter estimates by more than half across the corpus callosum. The exclusive use of longer diffusion times resulted in axon diameter estimates that were up to two times larger than those obtained with shorter diffusion times. Axon diameter and density maps appeared less noisy and showed improved contrast between different regions of the corpus callosum with higher maximum gradient strength. Known differences in axon diameter and density between the genu, body, and splenium of the corpus callosum were preserved and became more reproducible at higher maximum gradient strengths. Our results suggest that an optimal q-space sampling scheme for estimating in vivo axon diameters should incorporate the highest possible gradient strength. The improvement in axon diameter and density estimates that we demonstrate from increasing maximum gradient strength will inform protocol development and encourage the adoption of higher maximum gradient strengths for use in commercial human scanners.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
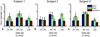

Similar articles
-
Estimation of the Mean Axon Diameter and Intra-axonal Space Volume Fraction of the Human Corpus Callosum: Diffusion q-space Imaging with Low q-values.Magn Reson Med Sci. 2016;15(1):83-93. doi: 10.2463/mrms.2014-0141. Epub 2015 Sep 4. Magn Reson Med Sci. 2016. PMID: 26346398
-
Age-related alterations in axonal microstructure in the corpus callosum measured by high-gradient diffusion MRI.Neuroimage. 2019 May 1;191:325-336. doi: 10.1016/j.neuroimage.2019.02.036. Epub 2019 Feb 18. Neuroimage. 2019. PMID: 30790671 Free PMC article.
-
High-gradient diffusion MRI reveals distinct estimates of axon diameter index within different white matter tracts in the in vivo human brain.Brain Struct Funct. 2020 May;225(4):1277-1291. doi: 10.1007/s00429-019-01961-2. Epub 2019 Sep 28. Brain Struct Funct. 2020. PMID: 31563995 Free PMC article.
-
Connectome 2.0: Developing the next-generation ultra-high gradient strength human MRI scanner for bridging studies of the micro-, meso- and macro-connectome.Neuroimage. 2021 Nov;243:118530. doi: 10.1016/j.neuroimage.2021.118530. Epub 2021 Aug 28. Neuroimage. 2021. PMID: 34464739 Free PMC article. Review.
-
Mapping the human connectome using diffusion MRI at 300 mT/m gradient strength: Methodological advances and scientific impact.Neuroimage. 2022 Jul 1;254:118958. doi: 10.1016/j.neuroimage.2022.118958. Epub 2022 Feb 23. Neuroimage. 2022. PMID: 35217204 Free PMC article. Review.
Cited by
-
In vivo mapping of human spinal cord microstructure at 300mT/m.Neuroimage. 2015 Sep;118:494-507. doi: 10.1016/j.neuroimage.2015.06.038. Epub 2015 Jun 19. Neuroimage. 2015. PMID: 26095093 Free PMC article.
-
Axonal damage in the optic radiation assessed by white matter tract integrity metrics is associated with retinal thinning in multiple sclerosis.Neuroimage Clin. 2020;27:102293. doi: 10.1016/j.nicl.2020.102293. Epub 2020 May 26. Neuroimage Clin. 2020. PMID: 32563921 Free PMC article.
-
Correlation between diffusion tensor indices and fascicular morphometric parameters of peripheral nerve.Front Physiol. 2023 Feb 23;14:1070227. doi: 10.3389/fphys.2023.1070227. eCollection 2023. Front Physiol. 2023. PMID: 36909220 Free PMC article.
-
MRI with ultrahigh field strength and high-performance gradients: challenges and opportunities for clinical neuroimaging at 7 T and beyond.Eur Radiol Exp. 2021 Aug 26;5(1):35. doi: 10.1186/s41747-021-00216-2. Eur Radiol Exp. 2021. PMID: 34435246 Free PMC article. Review.
-
Impact of transcytolemmal water exchange on estimates of tissue microstructural properties derived from diffusion MRI.Magn Reson Med. 2017 Jun;77(6):2239-2249. doi: 10.1002/mrm.26309. Epub 2016 Jun 25. Magn Reson Med. 2017. PMID: 27342260 Free PMC article.
References
-
- Hoffmeister B, Janig W, Lisney SJ. A proposed relationship between circumference and conduction velocity of unmyelinated axons from normal and regenerated cat hindlimb cutaneous nerves. Neuroscience. 1991;42(2):603–611. - PubMed
-
- Hursh JB. The properties of growing nerve fibers. American Journal of Physiology. 1939;127(1):140–153.
-
- Waxman SG, Kocsis JD, Stys PK. The Axon: Structure, Function and Pathophysiology. New York: Oxford University Press; 1995.
-
- Waxman SG. Physiology and Pathobiology of Axons. New York: Raven Press; 1978.
-
- Aboitiz F, Rodriguez E, Olivares R, Zaidel E. Age-related changes in fibre composition of the human corpus callosum: sex differences. Neuroreport. 1996;7(11):1761–1764. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

