Antibodies to the inositol 1,4,5-trisphosphate receptor type 1 (ITPR1) in cerebellar ataxia
- PMID: 25498830
- PMCID: PMC4300617
- DOI: 10.1186/s12974-014-0206-3
Antibodies to the inositol 1,4,5-trisphosphate receptor type 1 (ITPR1) in cerebellar ataxia
Abstract
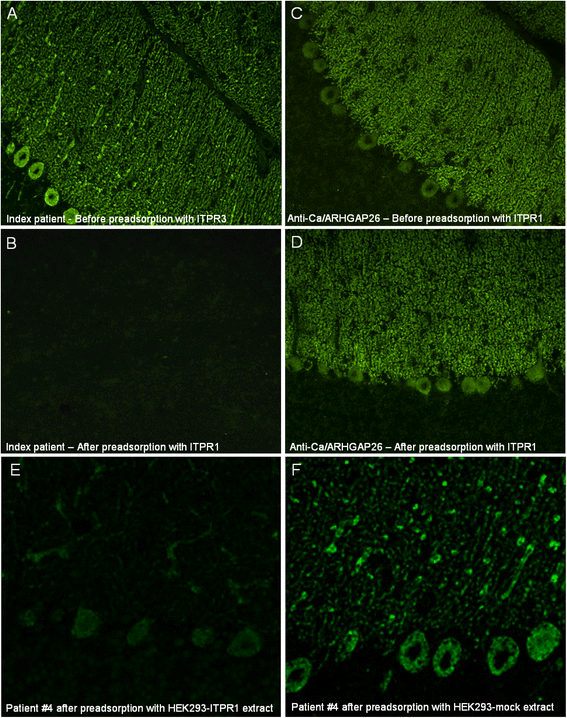
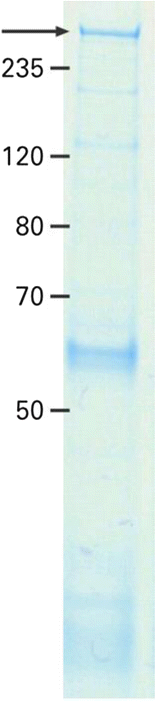
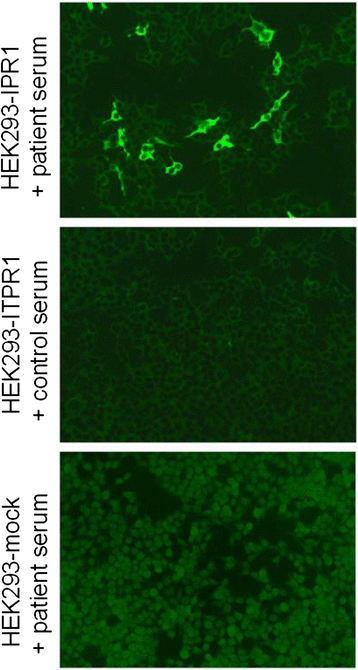
We report on a serum autoantibody associated with cerebellar ataxia. Immunohistochemical studies of sera from four patients referred for autoantibody testing revealed binding of high-titer (up to 1:5,000) IgG antibodies, mainly IgG1, to the molecular layer, Purkinje cell layer, and white matter on mouse, rat, porcine, and monkey cerebellum sections. The antibody bound to PC somata, dendrites, and axons, resulting in a binding pattern similar to that reported for anti-Ca/anti-ARHGAP26, but did not react with recombinant ARHGAP26. Extensive control studies were performed to rule out a broad panel of previously described paraneoplastic and non-paraneoplastic anti-neural autoantibodies. The characteristic binding pattern as well as double staining experiments suggested inositol 1,4,5-trisphosphate receptor type 1 (ITPR1) as the target antigen. Verification of the antigen included specific neutralization of the tissue reaction following preadsorption with ITPR1 (but not ARHGAP26) and a dot-blot assay with purified ITPR1 protein. By contrast, anti-ARHGAP26-positive sera did not bind to ITPR1. In a parallel approach, a combination of histoimmunoprecipitation and mass spectrometry also identified ITPR1 as the target antigen. Finally, a recombinant cell-based immunofluorescence assay using HEK293 cells expressing ITPR1 and ARHGAP26, respectively, confirmed the identification of ITPR1. Mutations of ITPR1 have previously been implicated in spinocerebellar ataxia with and without cognitive decline. Our findings suggest a role of autoimmunity against ITPR1 in the pathogenesis of autoimmune cerebellitis and extend the panel of diagnostic markers for this disease.
Figures
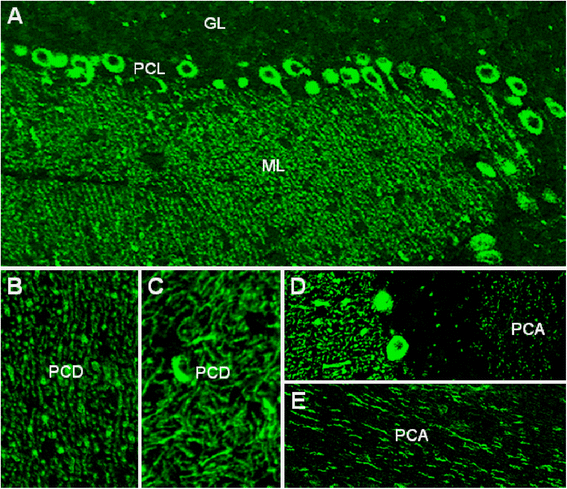
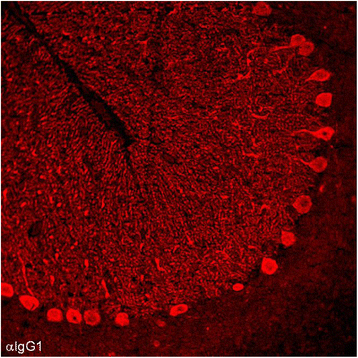
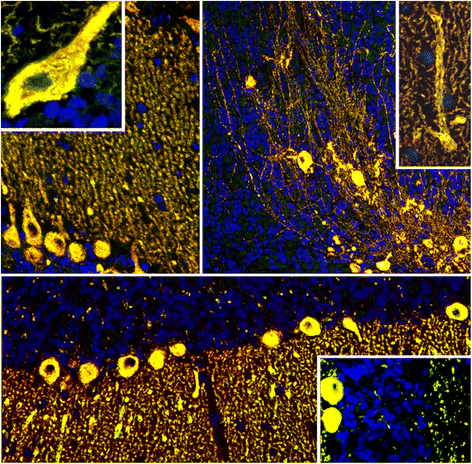

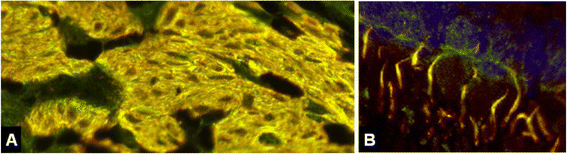

References
-
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler C, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

