Identification of N-{[6-chloro-4-(2,6-dimethoxyphenyl)quinazolin-2-yl]carbonyl}-l-leucine (NTRC-808), a novel nonpeptide chemotype selective for the neurotensin receptor type 2
- PMID: 25499438
- PMCID: PMC4296974
- DOI: 10.1016/j.bmcl.2014.11.047
Identification of N-{[6-chloro-4-(2,6-dimethoxyphenyl)quinazolin-2-yl]carbonyl}-l-leucine (NTRC-808), a novel nonpeptide chemotype selective for the neurotensin receptor type 2
Abstract
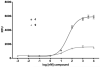
Compounds acting via the GPCR neurotensin receptor type 2 (NTS2) display analgesic effects in relevant animal models. Using a pharmacophore model based on known NT receptor nonpeptide compounds, we screened commercial databases to identify compounds that might possess activity at NTS2 receptor sites. Modification of our screening hit to include structural features known to be recognized by NTS1 and NTS2, led to the identification of the novel NTS2 selective nonpeptide, N-{[6-chloro-4-(2,6-dimethoxyphenyl)quinazolin-2-yl]carbonyl}-l-leucine (9). This compound is a potent partial agonist in the FLIPR assay with a profile of activity similar to that of the reference NTS2 analgesic nonpeptide levocabastine (5).
Keywords: FLIPR assay; Levocabastine; NTS2 receptor; Neurotensin; Pain; SR142948a; SR48692.
Copyright © 2014. Published by Elsevier Ltd.
Figures




References
-
- Caraway R, Leeman SE. J Biol Chem. 1973;248:6854. - PubMed
-
- Kitabgi P. Curr Opin Drug Discov Devel. 2002;5:764. - PubMed
-
- Sarret P, Beaudet A. In: Handbook of Chemical Neuroanatomy. Bjorkland A, Hokfelt T, Quinton P, editors. Vol. 20. Elsivier; Amsterdam: 2003. p. 323.
-
- Clineschmidt BV, McGuffin JC. Eur J Pharmacol. 1977;46:395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

