Altering the response to radiation: sensitizers and protectors
- PMID: 25499642
- PMCID: PMC4270009
- DOI: 10.1053/j.seminoncol.2014.09.013
Altering the response to radiation: sensitizers and protectors
Abstract
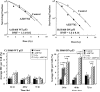
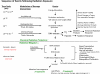
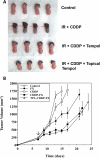
A number of agents are used clinically to enhance the efficacy of radiotherapy today, many of which are cytotoxic chemotherapies. Agents that enhance radiation induced tumor cell killing or protect normal tissues from the deleterious effects of ionizing radiation are collectively termed radiation modifiers. A significant effort in radiobiological research is geared towards describing and testing radiation modifiers with the intent of enhancing the therapeutic effects of radiation while minimizing normal tissue toxicity. In this review, we discuss the characteristics of these agents, the testing required to translate these agents into clinical trials, and highlight some challenges in these efforts.
Published by Elsevier Inc.
Figures




References
-
- Gilbert M. Radiotherapy in Hodgkin's Disease (malignant granulomatosis); anatomic and clinical foundations; governing principles, results. Am J Roentgenol. 1939;41:198–241.
-
- Peters M. A study in survivals in Hodgkin's disease treated radiologically. Am J Roentgenol. 1950;63:299–311.
-
- Coutard H. Roentgen therapy of epitheliomas of the tonsillar region, hypopharynx, anf larynx from 1920 to 1926. AJR Am J Roentgenol 1932. 28:313.
-
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. - PubMed
-
- Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

