Prostaglandin F2α receptor (FP) signaling regulates Bmp signaling and promotes chondrocyte differentiation
- PMID: 25499765
- PMCID: PMC4344833
- DOI: 10.1016/j.bbamcr.2014.12.003
Prostaglandin F2α receptor (FP) signaling regulates Bmp signaling and promotes chondrocyte differentiation
Abstract
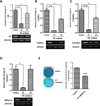
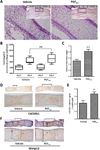
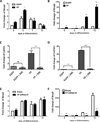
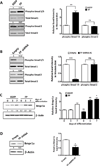
Prostaglandins are a group of lipid signaling molecules involved in various physiological processes. In addition, prostaglandins have been implicated in the development and progression of diseases including cancer, cardiovascular disease, and arthritis. Prostaglandins exert their effects through the activation of specific G protein-coupled receptors (GPCRs). In this report, we examined the role of prostaglandin F2α receptor (FP) signaling as a regulator of chondrocyte differentiation. We found that FP expression was dramatically induced during the differentiation of chondrocytes and was up-regulated in cartilages. Forced expression of FP in ATDC5 chondrogenic cell line resulted in the increased expression of differentiation-related genes and increased synthesis of the extracellular matrix (ECM) regardless of the presence of insulin. Similarly, PGF2α treatment induced the expression of chondrogenic marker genes. In contrast, knockdown of endogenous FP expression suppressed the expression of chondrocyte marker genes and ECM synthesis. Organ culture of cartilage rudiments revealed that PGF2α induces chondrocyte hypertrophy. Additionally, FP overexpression increased the levels of Bmp-6, phospho-Smad1/5, and Bmpr1a, while knockdown of FP reduced expression of those genes. These results demonstrate that up-regulation of FP expression plays an important role in chondrocyte differentiation and modulates Bmp signaling.
Keywords: Bmp; Chondrocytes; FP; Prostaglandin F(2α).
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures


References
-
- Kravis D, Upholt WB. Quantitation of type II procollagen mRNA levels during chick limb cartilage differentiation. Dev Biol. 1985;108:164–172. - PubMed
-
- O'Keefe RJ, Puzas JE, Loveys L, Hicks DG, Rosier RN. Analysis of type II and type X collagen synthesis in cultured growth plate chondrocytes by in situ hybridization: rapid induction of type X collagen in culture. J Bone Miner Res. 1994;9:1713–1722. - PubMed
-
- Wu CW, Tchetina EV, Mwale F, Hasty K, Pidoux I, Reiner A, Chen J, Van Wart HE, Poole AR. Proteolysis involving matrix metalloproteinase 13 (collagenase-3) is required for chondrocyte differentiation that is associated with matrix mineralization. J Bone Miner Res. 2002;17:639–651. - PubMed
-
- Mattot V, Raes MB, Henriet P, Eeckhout Y, Stehelin D, Vandenbunder B, Desbiens X. Expression of interstitial collagenase is restricted to skeletal tissue during mouse embryogenesis. J Cell Sci. 1995;108(Pt 2):529–535. - PubMed
-
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. The Journal of biological chemistry. 2007;282:11613–11617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

