Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections
- PMID: 25500204
- PMCID: PMC4869971
- DOI: 10.1136/gutjnl-2014-308043
Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections
Abstract
Background and aims: Peripancreatic fat necrosis occurs frequently in necrotising pancreatitis. Distinguishing markers from mediators of severe acute pancreatitis (SAP) is important since targeting mediators may improve outcomes. We evaluated potential agents in human pancreatic necrotic collections (NCs), pseudocysts (PCs) and pancreatic cystic neoplasms and used pancreatic acini, peripheral blood mononuclear cells (PBMC) and an acute pancreatitis (AP) model to determine SAP mediators.
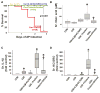
Methods: We measured acinar and PBMC injury induced by agents increased in NCs and PCs. Outcomes of caerulein pancreatitis were studied in lean rats coadministered interleukin (IL)-1β and keratinocyte chemoattractant/growth-regulated oncogene, triolein alone or with the lipase inhibitor orlistat.
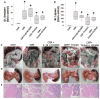
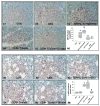
Results: NCs had higher fatty acids, IL-8 and IL-1β versus other fluids. Lipolysis of unsaturated triglyceride and resulting unsaturated fatty acids (UFA) oleic and linoleic acids induced necro-apoptosis at less than half the concentration in NCs but other agents did not do so at more than two times these concentrations. Cytokine coadministration resulted in higher pancreatic and lung inflammation than caerulein alone, but only triolein coadministration caused peripancreatic fat stranding, higher cytokines, UFAs, multisystem organ failure (MSOF) and mortality in 97% animals, which were prevented by orlistat.
Conclusions: UFAs, IL-1β and IL-8 are elevated in NCs. However, UFAs generated via peripancreatic fat lipolysis causes worse inflammation and MSOF, converting mild AP to SAP.
Keywords: OBESITY; PANCREATITIS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Conflict of interest statement
Figures

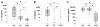



References
-
- Schaffler A, Hamer O, Dickopf J, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. Am J Gastroenterol. 2010;105:2474–84. - PubMed
-
- Schaffler A, Hamer OW, Dickopf J, et al. Admission visfatin levels predict pancreatic and peripancreatic necrosis in acute pancreatitis and correlate with clinical severity. Am J Gastroenterol. 2011;106:957–67. - PubMed
-
- Geokas MC, Rinderknecht H, Brodrick JW, et al. Studies on the ascites fluid of acute pancreatitis in man. Am J Dig Dis. 1978;23:182–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
