Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT
- PMID: 25500317
- PMCID: PMC4552933
- DOI: 10.1136/jnnp-2013-307149
Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT
Abstract
Objective: The approved use of onabotulinumtoxinA for prophylaxis of headaches in patients with chronic migraine (CM) involves treatment every 12 weeks. It is currently unknown whether patients who fail to respond to the first onabotulinumtoxinA treatment cycle will respond to subsequent treatment cycles. To help inform decisions about treating non-responders, we examined the probability of treatment cycle 1 non-responders responding in cycle 2, and cycle 1 and 2 non-responders responding in cycle 3.
Methods: Pooled PREEMPT data (two studies: a 24-week, 2-cycle, double-blind, randomised (1:1), placebo-controlled, parallel-group phase, followed by a 32-week, 3-cycle, open-label phase) evaluated onabotulinumtoxinA (155-195 U) for prophylaxis of headaches in persons with CM (≥15 days/month with headache ≥4 h/day). End points of interest included the proportion of study patients who first achieved a ≥50% reduction in headache days, moderate/severe headache days, total cumulative hours of headache on headache days, or a ≥5-point improvement in Headache Impact Test (HIT)-6. For treatment cycle 1, all eligible participants were included. For subsequent cycles, responders in a previous cycle were no longer considered first responders.
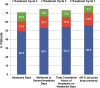
Results: Among onabotulinumtoxinA-treated patients (n=688) 49.3% had a ≥50% reduction in headache-day frequency during treatment cycle 1, with 11.3% and 10.3% of patients first responding during cycles 2 and 3, respectively. 54.2%, 11.6% and 7.4% of patients first responded with a ≥50% reduction in cumulative hours of headache, and 56.3%, 14.5% and 7.7% of patients first responded with a ≥5-point improvement in total HIT-6 during treatment cycles 1, 2 and 3, respectively.
Conclusions: A meaningful proportion of patients with CM treated with onabotulinumtoxinA who did not respond to the first treatment cycle responded in the second and third cycles of treatment.
Trial registration number: NCT00156910, NCT00168428.
Keywords: BOTULINUM TOXIN; HEADACHE; MIGRAINE.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
References
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia 2004;24(Suppl 1):9–160. - PubMed
-
- Bigal ME, Serrano D, Reed M, et al. . Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71:559–66. - PubMed
-
- Natoli J, Manack A, Dean B, et al. . Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010;30:599–609. - PubMed
-
- Olesen J; Headache Classification Committee of the International Headache Society (IHS) . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808. - PubMed
-
- Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med 2006;354:158–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
