Is there a common water-activity limit for the three domains of life?
- PMID: 25500507
- PMCID: PMC4438321
- DOI: 10.1038/ismej.2014.219
Is there a common water-activity limit for the three domains of life?
Abstract
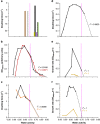
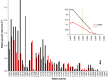
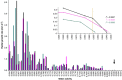
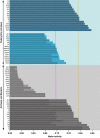
Archaea and Bacteria constitute a majority of life systems on Earth but have long been considered inferior to Eukarya in terms of solute tolerance. Whereas the most halophilic prokaryotes are known for an ability to multiply at saturated NaCl (water activity (a(w)) 0.755) some xerophilic fungi can germinate, usually at high-sugar concentrations, at values as low as 0.650-0.605 a(w). Here, we present evidence that halophilic prokayotes can grow down to water activities of <0.755 for Halanaerobium lacusrosei (0.748), Halobacterium strain 004.1 (0.728), Halobacterium sp. NRC-1 and Halococcus morrhuae (0.717), Haloquadratum walsbyi (0.709), Halococcus salifodinae (0.693), Halobacterium noricense (0.687), Natrinema pallidum (0.681) and haloarchaeal strains GN-2 and GN-5 (0.635 a(w)). Furthermore, extrapolation of growth curves (prone to giving conservative estimates) indicated theoretical minima down to 0.611 aw for extreme, obligately halophilic Archaea and Bacteria. These were compared with minima for the most solute-tolerant Bacteria in high-sugar (or other non-saline) media (Mycobacterium spp., Tetragenococcus halophilus, Saccharibacter floricola, Staphylococcus aureus and so on) and eukaryotic microbes in saline (Wallemia spp., Basipetospora halophila, Dunaliella spp. and so on) and high-sugar substrates (for example, Xeromyces bisporus, Zygosaccharomyces rouxii, Aspergillus and Eurotium spp.). We also manipulated the balance of chaotropic and kosmotropic stressors for the extreme, xerophilic fungi Aspergillus penicilloides and X. bisporus and, via this approach, their established water-activity limits for mycelial growth (∼0.65) were reduced to 0.640. Furthermore, extrapolations indicated theoretical limits of 0.632 and 0.636 a(w) for A. penicilloides and X. bisporus, respectively. Collectively, these findings suggest that there is a common water-activity limit that is determined by physicochemical constraints for the three domains of life.
Figures

References
-
- Andrews S, Pitt JI. Further studies on the water relations of xerophilic fungi, including some halophiles. J Gen Microbiol. 1987;133:233–238.
-
- Arai H. Foxing caused by fungi: twenty-five years of study. Int Biodeter Biodegr. 2000;46:181–188.
Publication types
MeSH terms
Substances
Grants and funding
- BB/F003471/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F00351X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBF/00351X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBF/003471/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

