The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases
- PMID: 25500872
- PMCID: PMC4571319
- DOI: 10.1038/aps.2014.121
The multiple universes of estrogen-related receptor α and γ in metabolic control and related diseases
Abstract
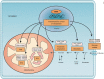
The identification of the estrogen-related receptors (ERRs) as the first orphan nuclear receptors ignited a new era in molecular endocrinology, which led to the discovery of new ligand-dependent response systems. Although ERR subfamily members have yet to be associated with a natural ligand, the characterization of these orphan receptors has demonstrated that they occupy a strategic node in the transcriptional control of cellular energy metabolism. In particular, ERRs are required for the response to various environmental challenges that require high energy levels by the organism. As central regulators of energy homeostasis, ERRs may also be implicated in the etiology of metabolic disorders, such as type 2 diabetes and metabolic syndrome. Here, we review the recent evidence that further highlights the role of ERRs in metabolic control, particularly in liver and skeletal muscle, and their likely involvement in metabolic diseases. Consequently, we also explore the promises and pitfalls of ERRs as potential therapeutic targets.
Figures

References
-
- Green S, Walter P, Kumar V, Krust A, Bornet JM, Argos P, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erbA. Nature (London) 1986; 320: 134–39. - PubMed
-
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science 1986; 231: 1150–54. - PubMed
-
- Giguère V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature 1988; 331: 91–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

