Targeting development of incretin-producing cells increases insulin secretion
- PMID: 25500886
- PMCID: PMC4382243
- DOI: 10.1172/JCI75838
Targeting development of incretin-producing cells increases insulin secretion
Abstract
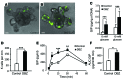
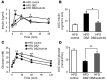
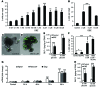
Glucagon-like peptide-1-based (GLP-1-based) therapies improve glycemic control in patients with type 2 diabetes. While these agents augment insulin secretion, they do not mimic the physiological meal-related rise and fall of GLP-1 concentrations. Here, we tested the hypothesis that increasing the number of intestinal L cells, which produce GLP-1, is an alternative strategy to augment insulin responses and improve glucose tolerance. Blocking the NOTCH signaling pathway with the γ-secretase inhibitor dibenzazepine increased the number of L cells in intestinal organoid-based mouse and human culture systems and augmented glucose-stimulated GLP-1 secretion. In a high-fat diet-fed mouse model of impaired glucose tolerance and type 2 diabetes, dibenzazepine administration increased L cell numbers in the intestine, improved the early insulin response to glucose, and restored glucose tolerance. Dibenzazepine also increased K cell numbers, resulting in increased gastric inhibitory polypeptide (GIP) secretion. Using a GLP-1 receptor antagonist, we determined that the insulinotropic effect of dibenzazepine was mediated through an increase in GLP-1 signaling. Together, our data indicate that modulation of the development of incretin-producing cells in the intestine has potential as a therapeutic strategy to improve glycemic control.
Figures


Comment in
-
Control of intestinal stem cell fate: A novel approach to treating diabetes.J Diabetes Investig. 2016 Mar;7(2):166-8. doi: 10.1111/jdi.12390. Epub 2015 Aug 9. J Diabetes Investig. 2016. PMID: 27042266 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

