TRPA1 is essential for the vascular response to environmental cold exposure
- PMID: 25501034
- PMCID: PMC4284811
- DOI: 10.1038/ncomms6732
TRPA1 is essential for the vascular response to environmental cold exposure
Abstract
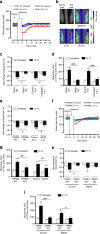
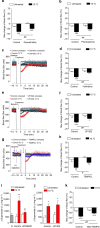
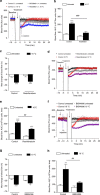
The cold-induced vascular response, consisting of vasoconstriction followed by vasodilatation, is critical for protecting the cutaneous tissues against cold injury. Whilst this physiological reflex response is historic knowledge, the mechanisms involved are unclear. Here by using a murine model of local environmental cold exposure, we show that TRPA1 acts as a primary vascular cold sensor, as determined through TRPA1 pharmacological antagonism or gene deletion. The initial cold-induced vasoconstriction is mediated via TRPA1-dependent superoxide production that stimulates α2C-adrenoceptors and Rho-kinase-mediated MLC phosphorylation, downstream of TRPA1 activation. The subsequent restorative blood flow component is also dependent on TRPA1 activation being mediated by sensory nerve-derived dilator neuropeptides CGRP and substance P, and also nNOS-derived NO. The results allow a new understanding of the importance of TRPA1 in cold exposure and provide impetus for further research into developing therapeutic agents aimed at the local protection of the skin in disease and adverse climates.
Figures


References
-
- Lewis T. Observations upon the reactions of the vessels of the human skin to cold. Heart 15, 177–208 (1930).
-
- Daanen H. A. Finger cold-induced vasodilation: a review. Eur. J. Appl. Physiol. 89, 411–426 (2003). - PubMed
-
- Johnson J. M., Yen T. C., Zhao K. & Kosiba W. A. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am. J. Physiol. Heart Circ. Physiol. 288, H1573–H1579 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

