Central nervous system effects of the second-generation antihistamines marketed in Japan--review of inter-drug differences using the proportional impairment ratio (PIR)-
- PMID: 25501360
- PMCID: PMC4264760
- DOI: 10.1371/journal.pone.0114336
Central nervous system effects of the second-generation antihistamines marketed in Japan--review of inter-drug differences using the proportional impairment ratio (PIR)-
Abstract
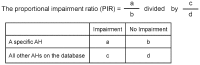
Background: Second-generation antihistamines (AHs) have, in general, fewer sedative effects than the first-generation. However, important inter-drug differences remain in the degree of cognitive and/or psychomotor impairment. The extent to which a particular compound causes disruption can be conveniently compared, to all other AHs, using the Proportional Impairment Ratio (PIR). Although the PIR can differentiate the relative impairment caused by individual drugs, there is no indication of the reliability of the ratios obtained.
Objective: To calculate the PIRs -together with 95% confidence intervals (CIs), as an index of reliability- and compare AHs currently, or soon to be, available in Japan, with respect to their intrinsic capacity to cause impairment.
Methods: Results from studies of cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine, mequitazine, and olopatadine were included in the PIR calculations. All data utilised came from crossover studies in healthy volunteers which were randomised and placebo and positive-internal controlled. Existing databases from studies reporting the sedative effects of AHs on objective (speed, accuracy, memory) and subjective (feeling) psychometrics were augmented, via results from suitable studies published after the previous reviews. The null value for a PIR was one.
Results: A total of 45 studies were finally included for this review. Of the AHs assessed, fexofenadine, ebastine, and levocetirizine showed a PIR for objective tests of 0. However, only fexofenadine (PIR = 0.49) had an upper limit of the 95% CI of less than 1. Fexofenadine, levocetirizine, desloratadine, olopatadine, loratadine, and mequitazine all had a PIR for subjective ratings of 0, but the upper limits of the 95% CIs were all in excess of 1, although fexofenadine (PIR = 2.57) was the lowest.
Conclusions: The results show that there are differences between second-generation AHs in the extent of sedation produced. However, subjective ratings indicate that patients may not necessarily be aware of this.
Conflict of interest statement
Figures



References
-
- Barbier AJ, Bradbury MJ (2007) Histaminergic control of sleep-wake cycles: recent therapeutic advances for sleep and wake disorders. CNS Neurol Disord Drug Targets 6:31–43. - PubMed
-
- Cookson J, Katona C, Taylor D (2002) The Use of Drugs in Psychiatry: The Evidence from Psychopharmacology, 5th ed. London: Gaskell Press.
-
- Passalacqua G, Scordamaglia A, Ruffoni S, Parodi MN, Canonica GW (1993) Sedation from H1 antagonists: evaluation methods and experimental results. Allergol Immunopathol (Madr) 21:79–83. - PubMed
-
- Shamsi Z, Hindmarch I (2000) Sedation and antihistamines: a review of inter-drug differences using proportional impairment ratios. Hum Psychopharmacol 15:S3–S30. - PubMed
-
- McDonald K, Trick L, Boyle J (2008) Sedation and antihistamines: an update. Review of inter-drug differences using proportional impairment ratios. Hum Psychopharmacol 23:555–570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

