Increased postexercise insulin sensitivity is accompanied by increased AS160 phosphorylation in slow-twitch soleus muscle
- PMID: 25501433
- PMCID: PMC4332192
- DOI: 10.14814/phy2.12162
Increased postexercise insulin sensitivity is accompanied by increased AS160 phosphorylation in slow-twitch soleus muscle
Abstract
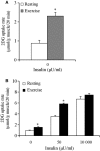
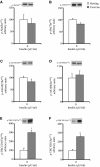
A single bout of exercise can enhance insulin-stimulated glucose uptake in both fast-twitch (type II) and slow-twitch (type I) skeletal muscle for several hours postexercise. Akt substrate of 160 kDa (AS160) is most distal insulin signaling proteins that have been proposed to contribute to the postexercise enhancement of insulin action in fast-twitch muscle. In this study, we examined whether the postexercise increase in insulin action of glucose uptake in slow-twitch muscle is accompanied by increased phosphorylation of AS160 and its paralog TBC1D1. Male Wistar rats (~1-month-old) were exercised on a treadmill for 180 min (9 m/min). Insulin (50 μU/mL)-stimulated glucose uptake was increased at 2 h after cessation of exercise in soleus muscle composed of predominantly slow-twitch fibers. This postexercise increase in insulin action of glucose uptake was accompanied by increased phosphorylation of AS160 (detected by phospho-Thr642 and phospho-Ser588 antibody). On the other hand, prior exercise did not increase phosphorylation of TBC1D1 (detected by phospho-Thr590) at 2 h postexercise. These results suggest the possibility that an enhancement in AS160 phosphorylation but not TBC1D1 phosphorylation is involved with increased postexercise insulin action of glucose uptake in slow-twitch muscle.
Keywords: AS160; TBC1D1; exercise; glucose uptake; insulin sensitivity; slow‐twitch muscle.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

References
-
- Arias E. B., Kim J., Funai K., Cartee G. D. 2007. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab.; 292:E1191-E1200. - PubMed
-
- Bonen A., Tan M. H., Clune P., Kirby R. L. 1985. Effects of exercise on insulin binding to human muscle. Am. J. Physiol. Endocrinol. Metab.; 248:E403-E408. - PubMed
-
- Bruss M. D., Arias E. B., Lienhard G. E., Cartee G. D. 2005. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes; 54:41-50. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

