Atmospheric hydrogen scavenging: from enzymes to ecosystems
- PMID: 25501483
- PMCID: PMC4309691
- DOI: 10.1128/AEM.03364-14
Atmospheric hydrogen scavenging: from enzymes to ecosystems
Abstract
We have known for 40 years that soils can consume the trace amounts of molecular hydrogen (H2) found in the Earth’s atmosphere.This process is predicted to be the most significant term in the global hydrogen cycle. However, the organisms and enzymes responsible for this process were only recently identified. Pure culture experiments demonstrated that several species of Actinobacteria, including streptomycetes and mycobacteria, can couple the oxidation of atmospheric H2 to the reduction of ambient O2. A combination of genetic, biochemical, and phenotypic studies suggest that these organisms primarily use this fuel source to sustain electron input into the respiratory chain during energy starvation. This process is mediated by a specialized enzyme, the group 5 [NiFe]-hydrogenase, which is unusual for its high affinity, oxygen insensitivity, and thermostability. Atmospheric hydrogen scavenging is a particularly dependable mode of energy generation, given both the ubiquity of the substrate and the stress tolerance of its catalyst. This minireview summarizes the recent progress in understanding how and why certain organisms scavenge atmospheric H2. In addition, it provides insight into the wider significance of hydrogen scavenging in global H2 cycling and soil microbial ecology.
Figures

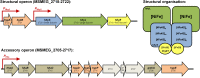
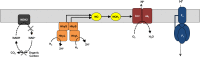
References
-
- Schmidt U. 1974. Molecular hydrogen in the atmosphere. Tellus A 26:78–90. doi:10.1111/j.2153-3490.1974.tb01954.x. - DOI
-
- Conrad R, Seiler W. 1980. Contribution of hydrogen production by biological nitrogen fixation to the global hydrogen budget. J Geophys Res 85:5493–5498. doi:10.1029/JC085iC10p05493. - DOI
-
- Ehhalt DH, Rohrer F. 2009. The tropospheric cycle of H2: a critical review. Tellus B 61:500–535. doi:10.1111/j.1600-0889.2009.00416.x. - DOI
-
- Novelli PC, Lang PM, Masarie A, Hurst DF, Elkins W. 1999. Molecular hydrogen in the troposphere: global distribution and budget. J Geophys Res Atmos 104:30427–30444. doi:10.1029/1999JD900788. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

