Metals in cyanobacteria: analysis of the copper, nickel, cobalt and arsenic homeostasis mechanisms
- PMID: 25501581
- PMCID: PMC4284471
- DOI: 10.3390/life4040865
Metals in cyanobacteria: analysis of the copper, nickel, cobalt and arsenic homeostasis mechanisms
Abstract
Traces of metal are required for fundamental biochemical processes, such as photosynthesis and respiration. Cyanobacteria metal homeostasis acquires an important role because the photosynthetic machinery imposes a high demand for metals, making them a limiting factor for cyanobacteria, especially in the open oceans. On the other hand, in the last two centuries, the metal concentrations in marine environments and lake sediments have increased as a result of several industrial activities. In all cases, cells have to tightly regulate uptake to maintain their intracellular concentrations below toxic levels. Mechanisms to obtain metal under limiting conditions and to protect cells from an excess of metals are present in cyanobacteria. Understanding metal homeostasis in cyanobacteria and the proteins involved will help to evaluate the use of these microorganisms in metal bioremediation. Furthermore, it will also help to understand how metal availability impacts primary production in the oceans. In this review, we will focus on copper, nickel, cobalt and arsenic (a toxic metalloid) metabolism, which has been mainly analyzed in model cyanobacterium Synechocystis sp. PCC 6803.
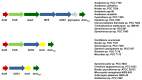
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

