BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells
- PMID: 25501832
- PMCID: PMC4649848
- DOI: 10.1038/cddis.2014.540
BMPR2 inhibition induced apoptosis and autophagy via destabilization of XIAP in human chondrosarcoma cells
Abstract
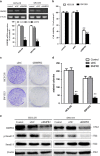
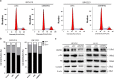
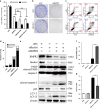
Bone morphogenetic proteins (BMPs) are multifunctional proteins, and their receptors (BMPRs) have crucial roles in the process of signaling. However, their function in cancer is somewhat inconsistent. It has been demonstrated that more prevalent expression of bone morphogenetic protein receptor 2 (BMPR2) has been detected in dedifferentiated chondrosarcomas than conventional chondrosarcomas. Here, we find that BMPR2 inhibition induces apoptosis and autophagy of chondrosarcoma. We found that BMPR2 expression was correlated with the clinicopathological features of chondrosarcomas, and could predict the treatment outcome. Knockdown of BMPR2 by small interfering RNA results in growth inhibition in chondrosarcoma cells. Silencing BMPR2 promoted G2/M cell cycle arrest, induced chondrosarcoma cell apoptosis through caspase-3-dependent pathway via repression of X-linked inhibitor of apoptosis protein (XIAP) and induced autophagy of chondrosarcoma cells via XIAP-Mdm2-p53 pathway. Inhibition of autophagy induced by BMPR2 small interfering RNA (siBMPR2) sensitized chondrosarcoma cells to siBMPR2-induced apoptotic cell death, suggesting that autophagy has a protective role for chondrosarcoma cells in context of siBMPR2-induced apoptotic cell death. In vivo tumorigenicity assay in mice indicated that inhibition of BMPR2 reduced tumor growth. Taken together, our results suggest that BMPR2 has a significant role in the tumorigenesis of chondrosarcoma, and could be an important prognostic marker for chondrosarcoma. BMPR2 inhibition could eventually provide a promising therapy for chondrosarcoma treatment.
Figures





References
-
- 1O'Neal LW, Ackerman LV. Chondrosarcoma of bone. Cancer 1952; 5: 551–577. - PubMed
-
- 2Bauer HC, Brosjo O, Kreicbergs A, Lindholm J. Low risk of recurrence of enchondroma and low-grade chondrosarcoma in extremities. 80 patients followed for 2–25 years. Acta Orthop Scand 1995; 66: 283–288. - PubMed
-
- 3Eriksson AI, Schiller A, Mankin HJ. The management of chondrosarcoma of bone. Clin Orthop Relat Res 1980; 153: 44–66. - PubMed
-
- 4Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE et al. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am 1999; 81: 326–338. - PubMed
-
- 5Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol 2005; 21: 659–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

