Au-nanomaterials as a superior choice for near-infrared photothermal therapy
- PMID: 25501919
- PMCID: PMC6270707
- DOI: 10.3390/molecules191220580
Au-nanomaterials as a superior choice for near-infrared photothermal therapy
Abstract
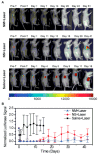
Photothermal therapy (PPT) is a platform to fight cancer by using multiplexed interactive plasmonic nanomaterials as probes in combination with the excellent therapeutic performance of near-infrared (NIR) light. With recent rapid developments in optics and nanotechnology, plasmonic materials have potential in cancer diagnosis and treatment, but there are some concerns regarding their clinical use. The primary concerns include the design of plasmonic nanomaterials which are taken up by the tissues, perform their function and then clear out from the body. Gold nanoparticles (Au NPs) can be developed in different morphologies and functionalized to assist the photothermal therapy in a way that they have clinical value. This review outlines the diverse Au morphologies, their distinctive characteristics, concerns and limitations to provide an idea of the requirements in the field of NIR-based therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

