Early mesozoic coexistence of amniotes and hepadnaviridae
- PMID: 25501991
- PMCID: PMC4263362
- DOI: 10.1371/journal.pgen.1004559
Early mesozoic coexistence of amniotes and hepadnaviridae
Abstract
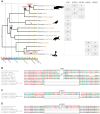
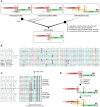
Hepadnaviridae are double-stranded DNA viruses that infect some species of birds and mammals. This includes humans, where hepatitis B viruses (HBVs) are prevalent pathogens in considerable parts of the global population. Recently, endogenized sequences of HBVs (eHBVs) have been discovered in bird genomes where they constitute direct evidence for the coexistence of these viruses and their hosts from the late Mesozoic until present. Nevertheless, virtually nothing is known about the ancient host range of this virus family in other animals. Here we report the first eHBVs from crocodilian, snake, and turtle genomes, including a turtle eHBV that endogenized >207 million years ago. This genomic "fossil" is >125 million years older than the oldest avian eHBV and provides the first direct evidence that Hepadnaviridae already existed during the Early Mesozoic. This implies that the Mesozoic fossil record of HBV infection spans three of the five major groups of land vertebrates, namely birds, crocodilians, and turtles. We show that the deep phylogenetic relationships of HBVs are largely congruent with the deep phylogeny of their amniote hosts, which suggests an ancient amniote-HBV coexistence and codivergence, at least since the Early Mesozoic. Notably, the organization of overlapping genes as well as the structure of elements involved in viral replication has remained highly conserved among HBVs along that time span, except for the presence of the X gene. We provide multiple lines of evidence that the tumor-promoting X protein of mammalian HBVs lacks a homolog in all other hepadnaviruses and propose a novel scenario for the emergence of X via segmental duplication and overprinting of pre-existing reading frames in the ancestor of mammalian HBVs. Our study reveals an unforeseen host range of prehistoric HBVs and provides novel insights into the genome evolution of hepadnaviruses throughout their long-lasting association with amniote hosts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

