Use of the Fluocinolone Acetonide Intravitreal Implant for the Treatment of Noninfectious Posterior Uveitis: 3-Year Results of a Randomized Clinical Trial in a Predominantly Asian Population
- PMID: 25502122
- PMCID: PMC4470982
- DOI: 10.1007/s40123-014-0027-6
Use of the Fluocinolone Acetonide Intravitreal Implant for the Treatment of Noninfectious Posterior Uveitis: 3-Year Results of a Randomized Clinical Trial in a Predominantly Asian Population
Abstract
Introduction: The fluocinolone acetonide (FA) intravitreal implant 0.59 mg (Retisert(®), Bausch + Lomb, Rochester, NY, USA) provides sustained release of FA directly to the vitreous cavity over a prolonged period of time. The purpose of this study was to evaluate the safety and efficacy of a 0.59- and 2.1-mg FA intravitreal implant in patients with noninfectious posterior uveitis.
Methods: A prospective, multicenter, randomized, double-masked, dose-controlled study was performed. Patients were randomized to the 0.59- or 2.1-mg FA implant surgically placed in the vitreous cavity through a pars plana incision and were evaluated at visits through 3 years. Patients with bilateral disease had the more severely affected eye implanted. Outcomes included uveitis recurrence rate, best-corrected visual acuity (BCVA), use of adjunctive therapy, and safety.
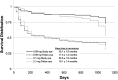
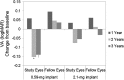
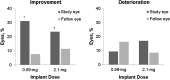
Results: A total of 239 patients, predominantly Asian, were implanted (n = 117, 0.59-mg implant; n = 122, 2.1-mg implant). Approximately 80% of patients had bilateral disease. Recurrence rates for implanted eyes decreased from 42.3% during the 1-year pre-implantation period to 25.9% during the 3-year post-implantation period (P = 0.0003) and increased for nonimplanted fellow eyes from 19.8 to 59.7% (P < 0.0001). More implanted eyes gained ≥3 lines of BCVA compared to nonimplanted fellow eyes (P ≤ 0.0046); and implanted eyes required less adjunctive systemic therapy and fewer periocular injections (P < 0.0001). Elevations of intraocular pressure (≥10 mm Hg) were frequent in implanted eyes (67.8%, 0.59-mg implant; 71.3%, 2.1-mg implant); nearly all (94.9%) phakic implanted eyes required cataract surgery.
Conclusion: The FA intravitreal implant significantly reduced uveitis recurrence rates and led to improvements in visual acuity and reductions in adjunctive therapy. Lens clarity and intraocular pressure require monitoring.
Conflict of interest statement
Virender S. Sangwan and P. Andrew Pearson declare no conflict of interest. Hemanth Paul was an employee of Bausch + Lomb at the time of the study. Timothy Comstock was an employee of Bausch + Lomb at the time of the study.
Figures
References
-
- Forster DJ. General approach to the uveitis patient and treatment strategies. In: Yanoff M, Duker JS, editors. Ophthalmology. 2. St. Louis: Mosby; 2004. pp. 1115–1120.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

