Trigonal scaffolds for multivalent targeting of melanocortin receptors
- PMID: 25502141
- PMCID: PMC4780440
- DOI: 10.1039/c4ob02094d
Trigonal scaffolds for multivalent targeting of melanocortin receptors
Abstract
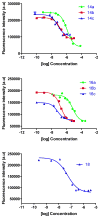
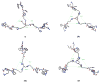
Melanocortin receptors can be used as biomarkers to detect and possibly treat melanoma. To these ends, molecules bearing one, two, or three copies of the weakly binding ligand MSH(4) were attached to scaffolds based on phloroglucinol, tripropargylamine, and 1,4,7-triazacyclononane by means of the copper-assisted azide-alkyne cyclization. This synthetic design allows rapid assembly of multivalent molecules. The bioactivities of these compounds were evaluated using a competitive binding assay that employed human embryonic kidney cells engineered to overexpress the melanocortin 4 receptor. The divalent molecules exhibited 10- to 30-fold higher levels of inhibition when compared to the corresponding monovalent molecules, consistent with divalent binding. The trivalent molecules were only statistically (∼2-fold) better than the divalent molecules, still consistent with divalent binding but inconsistent with trivalent binding. Possible reasons for these behaviors and planned refinements of the multivalent constructs targeting melanocortin receptors based on these scaffolds are discussed.
Figures


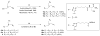
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

