Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation
- PMID: 25502213
- PMCID: PMC4716621
- DOI: 10.1038/cmi.2014.116
Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation
Abstract
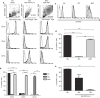
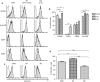
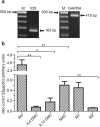
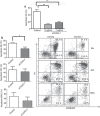
Circulating monocytes (Mos) may continuously repopulate macrophage (MAC) or dendritic cell (DC) populations to maintain homeostasis. MACs and DCs are specialized cells that play different and complementary immunological functions. Accordingly, they present distinct migratory properties. Specifically, whereas MACs largely remain in tissues, DCs are capable of migrating from peripheral tissues to lymphoid organs. The aim of this work was to analyze the expression of the fractalkine receptor (CX3CR1) during the monocytic differentiation process. Freshly isolated Mos express high levels of both CX3CR1 mRNA and protein. During the Mo differentiation process, CX3CR1 is downregulated in both DCs and MACs. However, MACs showed significantly higher CX3CR1 expression levels than did DC. We also observed an antagonistic CX3CR1 regulation by interferon (IFN)-γ and interleukin (IL)-4 during MAC activation through the classical and alternative MAC pathways, respectively. IFN-γ inhibited the loss of CX3CR1, but IL-4 induced it. Additionally, we demonstrated an association between CX3CR1 expression and apoptosis prevention by soluble fractalkine (sCX3CL1) in Mos, DCs and MACs. This is the first report demonstrating sequential and differential CX3CR1 modulation during Mo differentiation. Most importantly, we demonstrated a functional link between CX3CR1 expression and cell survival in the presence of sCX3CL1.
Figures

References
-
- 1Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 2006; 311: 83–87. - PubMed
-
- 2Whitelaw DM. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet 1972; 5: 311–317. - PubMed
-
- 3Ziegler-Heitbrock HW. Definition of human blood monocytes. J Leukoc Biol 2000; 67: 603–606. - PubMed
-
- 4Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006; 211: 609–618. - PubMed
-
- 5Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells. Semin Immunol 2005; 17: 313–318. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

