Anti-inflammatory and anti-fibrotic profile of fish oil emulsions used in parenteral nutrition-associated liver disease
- PMID: 25502575
- PMCID: PMC4264955
- DOI: 10.1371/journal.pone.0115404
Anti-inflammatory and anti-fibrotic profile of fish oil emulsions used in parenteral nutrition-associated liver disease
Abstract
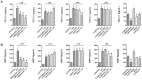
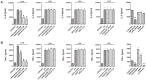
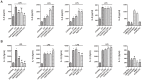
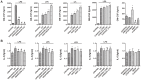
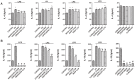
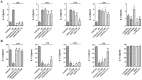
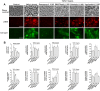
Home parenteral nutrition (PN) is associated with many complications including severe hepatobiliary dysfunction. Commercial ω-6 fatty acid-soybean based-lipid emulsions in PN may mediate long term PN associate liver disease (PNALD) whereas ω-3-fish oil parenteral emulsions have shown to reverse PNALD in children. However, its clinical effectiveness in adults has been scarcely reported. In this work, we study the role of soybean and fish oil lipid commercial emulsions on inflammatory and profibrotic liver markers in adults with long term PNALD and in in vitro cellular models. Inflammatory and profibrotic markers were measured in serum of ten adults with long term PNALD and in culture supernatants of monocytes. Liver epithelial to mesenchymal transition (EMT) was induced by transforming growth factor beta 1 (TGFβ1) to evaluate in vitro liver fibrosis. Omegaven®, a 100% fish oil commercial emulsion, was infused during four months in two patients with severe long term PNALD reversing, at the first month, the inflammatory, profibrotic and clinical parameters of PNALD. The effect was maintained during the treatment course but impaired when conventional lipid emulsions were reintroduced. The other patients under chronic soybean oil-based PN showed elevated inflammatory and profibrotic parameters. In vitro human monocytes stimulated with lipopolysaccharide induced a strong inflammatory response that was suppressed by Omegaven®, but increased by soybean emulsions. In other experiments, TGFβ1 induced EMT that was suppressed by Omegaven® and enhanced by soybean oil lipid emulsions. Omegaven® improves clinical, anti-inflammatory and anti-fibrotic parameters in adults with long-term home PNALD.
Conflict of interest statement
Figures




Similar articles
-
Reversal of parenteral nutrition-associated liver disease with a fish oil-based lipid emulsion (Omegaven) in an adult dependent on home parenteral nutrition.JPEN J Parenter Enteral Nutr. 2013 Mar;37(2):274-80. doi: 10.1177/0148607112450301. Epub 2012 Jun 8. JPEN J Parenter Enteral Nutr. 2013. PMID: 22683685
-
Parenteral Soy Oil and Fish Oil Emulsions: Impact of Dose Restriction on Bile Flow and Brain Size of Parenteral Nutrition-Fed Neonatal Piglets.JPEN J Parenter Enteral Nutr. 2015 Aug;39(6):677-87. doi: 10.1177/0148607114556494. Epub 2014 Oct 17. JPEN J Parenter Enteral Nutr. 2015. PMID: 25326097
-
Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience.JPEN J Parenter Enteral Nutr. 2009 Sep-Oct;33(5):541-7. doi: 10.1177/0148607109332773. Epub 2009 Jul 1. JPEN J Parenter Enteral Nutr. 2009. PMID: 19571170 Review.
-
Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease.Prostaglandins Leukot Essent Fatty Acids. 2013 Sep;89(4):153-8. doi: 10.1016/j.plefa.2013.02.008. Epub 2013 Apr 18. Prostaglandins Leukot Essent Fatty Acids. 2013. PMID: 23602846 Review.
-
Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil.Curr Opin Organ Transplant. 2010 Jun;15(3):334-40. doi: 10.1097/mot.0b013e3283394879. Curr Opin Organ Transplant. 2010. PMID: 20503524 Review.
Cited by
-
Fish Oil Emulsion Reduces Liver Injury and Liver Transplantation in Children with Intestinal Failure-Associated Liver Disease: A Multicenter Integrated Study.J Pediatr. 2021 Mar;230:46-54.e2. doi: 10.1016/j.jpeds.2020.09.068. Epub 2020 Oct 8. J Pediatr. 2021. PMID: 33038344 Free PMC article.
-
Effect of Polymeric Nanoparticles with Entrapped Fish Oil or Mupirocin on Skin Wound Healing Using a Porcine Model.Int J Mol Sci. 2022 Jul 11;23(14):7663. doi: 10.3390/ijms23147663. Int J Mol Sci. 2022. PMID: 35887016 Free PMC article.
-
High Dose Intravenous Fish Oil Reduces Inflammation-A Retrospective Tale from Two Centers.Nutrients. 2020 Sep 19;12(9):2865. doi: 10.3390/nu12092865. Nutrients. 2020. PMID: 32961695 Free PMC article.
-
Development and Validation of In Vitro Assessment Protocol of Novel Intravenous Nanoemulsions for Parenteral Nutrition.Pharmaceutics. 2025 Apr 8;17(4):493. doi: 10.3390/pharmaceutics17040493. Pharmaceutics. 2025. PMID: 40284488 Free PMC article.
-
Intravenous Lipid Emulsions in Parenteral Nutrition.Adv Nutr. 2015 Sep 15;6(5):600-10. doi: 10.3945/an.115.009084. Print 2015 Sep. Adv Nutr. 2015. PMID: 26374182 Free PMC article. Review.
References
-
- Dudrick SJ (2003) Early developments and clinical applications of total parenteral nutrition. JPEN J Parenter Enteral Nutr 27:291–299. - PubMed
-
- Carter BA, Karpen SJ (2007) Intestinal failure-associated liver disease: management and treatment strategies past, present, and future. Semin Liver Dis 27:251–258. - PubMed
-
- Kelly DA (1998) Liver complications of pediatric parenteral nutrition—epidemiology. Nutrition 14:153–157. - PubMed
-
- Chan S, McCowen KC, Bistrian BR, Thibault A, Keane-Ellison M, et al. (1999) Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery 126:28–34. - PubMed
-
- Clayton PT, Bowron A, Mills KA, Massoud A, Casteels M, et al. (1993) Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology 105:1806–1813. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

