A mixed-methods feasibility trial of protein kinase C iota inhibition with auranofin in asymptomatic ovarian cancer patients
- PMID: 25502607
- PMCID: PMC4536897
- DOI: 10.1159/000369257
A mixed-methods feasibility trial of protein kinase C iota inhibition with auranofin in asymptomatic ovarian cancer patients
Abstract
Purpose: This trial was undertaken (1) to determine the feasibility of enrolling asymptomatic ovarian cancer patients with CA-125 elevation in a trial with the protein kinase C iota (PKCι) inhibitor auranofin and (2) to understand patients' perceptions of CA-125 monitoring.
Methods: Asymptomatic ovarian cancer patients with CA-125 elevation received 3 mg auranofin orally twice per day and were evaluated. The patients participated in interviews about CA-125 monitoring.
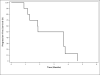
Results: Ten patients were enrolled in slightly over 6 months, exceeding our anticipated accrual rate. Four manifested stable CA-125 levels for 1 month or longer. The median progression-free survival was 2.8 months (95% CI: 1.3-3.8); auranofin was well tolerated. One patient had baseline and monthly CA-125 levels of 5,570, 6,085, 3,511, and 2,230 U/ml, respectively, stopped auranofin because of radiographic progression at 3 months, and manifested an increase in CA-125 to 7,168 U/ml approximately 3 months later. Patient interviews revealed (1) the important role of CA-125 in cancer monitoring, (2) ardent advocacy of CA-125 testing, and (3) an evolution toward CA-125 assuming a life of its own.
Conclusions: This study showed the feasibility of enrolling asymptomatic ovarian cancer patients with CA-125 elevation in a trial with auranofin. One patient had a decline in CA-125, suggesting that PKCι inhibition merits further study in ovarian cancer.
© 2014 S. Karger AG, Basel.
Figures

References
-
- Zhang L, Huang J, Yang N, et al. Integrative genomic analysis of protein kinase C family identified PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006;66:4627–35. - PubMed
-
- Syrios J, Banerjee S Kaye SB. Advanced epithelial ovarian cancer: from standard chemotherapy to promising molecular pathway targets – where are we now? Anticancer Res. 2014;34:2069–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

