Arteries are formed by vein-derived endothelial tip cells
- PMID: 25502622
- PMCID: PMC4275597
- DOI: 10.1038/ncomms6758
Arteries are formed by vein-derived endothelial tip cells
Abstract
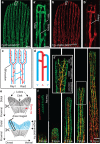
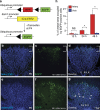
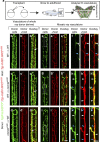
Tissue vascularization entails the formation of a blood vessel plexus, which remodels into arteries and veins. Here we show, by using time-lapse imaging of zebrafish fin regeneration and genetic lineage tracing of endothelial cells in the mouse retina, that vein-derived endothelial tip cells contribute to emerging arteries. Our movies uncover that arterial-fated tip cells change migration direction and migrate backwards within the expanding vascular plexus. This behaviour critically depends on chemokine receptor cxcr4a function. We show that the relevant Cxcr4a ligand Cxcl12a selectively accumulates in newly forming bone tissue even when ubiquitously overexpressed, pointing towards a tissue-intrinsic mode of chemokine gradient formation. Furthermore, we find that cxcr4a mutant cells can contribute to developing arteries when in association with wild-type cells, suggesting collective migration of endothelial cells. Together, our findings reveal specific cell migratory behaviours in the developing blood vessel plexus and uncover a conserved mode of artery formation.
Figures




References
-
- Eilken H. M. & Adams R. H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell. Biol. 22, 617–625 (2010). - PubMed
-
- Potente M., Gerhardt H. & Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 146, 873–887 (2011). - PubMed
-
- Wacker A. & Gerhardt H. Endothelial development taking shape. Curr. Opin. Cell. Biol. 23, 676–685 (2011). - PubMed
-
- DiPietro L. A. Angiogenesis and scar formation in healing wounds. Curr. Opin. Rheumatol. 25, 87–91 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

