An NMR-based metabolomic approach to investigate the effects of supplementation with glutamic acid in piglets challenged with deoxynivalenol
- PMID: 25502722
- PMCID: PMC4263475
- DOI: 10.1371/journal.pone.0113687
An NMR-based metabolomic approach to investigate the effects of supplementation with glutamic acid in piglets challenged with deoxynivalenol
Abstract
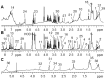
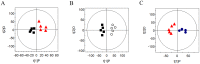
Deoxynivalenol (DON) has various toxicological effects in humans and pigs that result from the ingestion of contaminated cereal products. This study was conducted to investigate the protective effects of dietary supplementation with glutamic acid on piglets challenged with DON. A total of 20 piglets weaned at 28 d of age were randomly assigned to receive 1 of 4 treatments (5 piglets/treatment): 1) basal diet, negative control (NC); 2) basal diet +4 mg/kg DON (DON); 3) basal diet +2% (g/g) glutamic acid (GLU); 4) basal diet +4 mg/kg DON +2% glutamic acid (DG). A 7-d adaptation period was followed by 30 days of treatment. A metabolite analysis using nuclear magnetic resonance spectroscopy (1H-NMR)-based metabolomic technology and the determination of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities for plasma, as well as the activity of Caspase-3 and the proliferation of epithelial cells were conducted. The results showed that contents of low-density lipoprotein, alanine, arginine, acetate, glycoprotein, trimethylamine-N-oxide (TMAO), glycine, lactate, and urea, as well as the glutamate/creatinine ratio were higher but high-density lipoprotein, proline, citrate, choline, unsaturated lipids and fumarate were lower in piglets of DON treatment than that of NC treatment (P<0.05). Compared with DON treatment, dietary supplementation with glutamic acid increased the plasma concentrations of proline, citrate, creatinine, unsaturated lipids, and fumarate, and decreased the concentrations of alanine, glycoprotein, TMAO, glycine, and lactate, as well as the glutamate/creatinine ratio (P<0.05). Addition glutamic acid to DON treatment increased the plasma activities of SOD and GSH-Px and the proliferating cell nuclear antigen (PCNA) labeling indexes for the jejunum and ileum (P<0.05). These novel findings indicate that glutamic acid has the potential to repair the injuries associated with oxidative stress as well as the disturbances of energy and amino acid metabolism induced by DON.
Conflict of interest statement
Figures

References
-
- Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Archives of Toxicology 84:663–679. - PubMed
-
- Schollenberger M, Jara HT, Suchy S, Drochner W, Muller HM (2002) Fusarium toxins in wheat flour collected in an area in southwest Germany. International Journal of Food Microbiology 72:85–89. - PubMed
-
- He P, Young LG, Forsberg C (1993) Microbially Detoxified Vomitoxin-Contaminated Corn for Young-Pigs. Journal of Animal Science 71:963–967. - PubMed
-
- Awad WA, Ghareeb K, Bohm J, Zentek J (2010) Decontamination and detoxification strategies for the Fusarium mycotoxin deoxynivalenol in animal feed and the effectiveness of microbial biodegradation. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment 27:510–520. - PubMed
-
- Volkl A, Vogler B, Schollenberger M, Karlovsky P (2004) Microbial detoxification of mycotoxin deoxynivalenol. Journal of Basic Microbiology 44:147–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

